Reports: AC4
48016-AC4 New Photoremovable Protecting Groups with the Capacity to Release Alcohols in High Quantum Yields
Photoremovable protecting groups (PRPGs) have been used in a wide variety of applications. Their utility has spanned a wide variety of areas, including the release of fragrances from household goods, aiding in multi-step syntheses and drug and gene delivery. PRPGs also allow biochemists to release bioactive compounds in living tissue with both high temporal and spatial accuracy, thus making it possible to study physiological events such as enzyme activity and ion channel permeability. The above examples represent only fractions of the photoremovable protecting groups that are being developed and used for applications; thus, it is clear that the choice of photoremovable protecting group is critical, depends on the system under investigation, and must be tailored to each application. Thus, there is a need for new PRPGs that can satisfy the requirements of diverse applications. Understanding mechanistic details makes it easier to select the most suitable PRPG for each specific application and to design new PRPGs that can be tailored to specific applications.
The major objective of this grant is to design new PRGPs with novel physical properties. Our goal is to use intramolecular H-atom bonding and irreversible cleavage reactions to control the rate of release and the quantum yield for release.
Our major findings are the following:
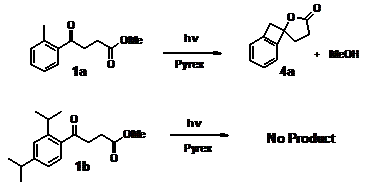
1. In our continuing effort to design new photoremovable protecting groups with unique properties, we synthesized 1a and 1b, which differ from the benzophenone based PRGPs we studied previously because 1a and 1b are more flexible. 1a and 1b are butyrophoneone derivatives and thus have a flexible alkyl chain. We investigated the photoreactivity of 1a and 1b and how their flexibility affects the mechanisms for photorelease.
Scheme 1.
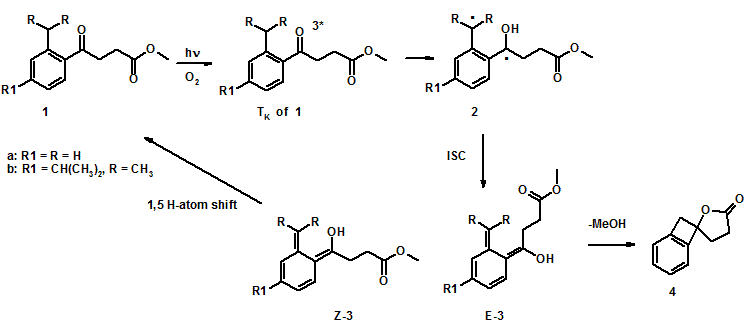
Ester 1a released its alcohol moiety upon exposure to light, whereas 1b was photostable (Scheme 1). Laser flash photolysis shows that irradiation of 1a results in formation of biradicals 2a via intramolecular H-atom abstraction. Biradical 2a (t = 580 ns, methanol) undergoes intersystem crossing to form Z-3a and E-3a. Z-3a is short-lived (t ~ 80 ms, in methanol) as it undergoes 1,5 H-atom shift to regenerate the starting material, whereas E-3a has a longer lifetime (~500 ms, methanol) and lactonizes to release its alcohol moiety (Scheme 2). Similarly, irradiation of 1b yields 2b (t = 1.9 ms, methanol) that is considerably longer-lived than 2a is. However, 2b only undergoes intersystem crossing to form the Z-3b (t = 6 ms, methanol), which regenerates 1b. Calculations show that the necessary rotation in 2b to form the E-3b is hindered; thus, 2b forms only Z-3b.
Scheme 2.
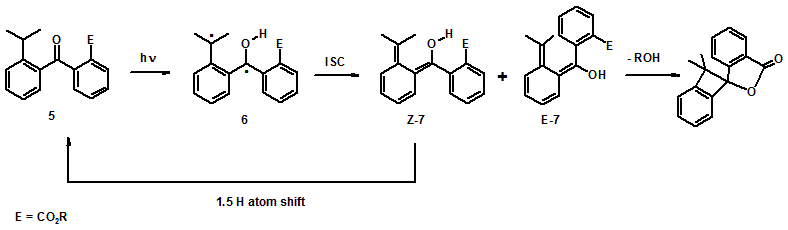
We have previously shown that 5 releases its alcohol moiety by photoenolization (Scheme 3). The release from 5 is independent of the reaction medium and is not quenched by molecular oxygen, but the E-photoenol (E-7) from 5 has a lifetime on the order of seconds; thus, the rate of release from E-7 is much slower than that observed for E-3a. The rate of release from 1a is presumably the same as the rate of decay of photoenol E-3a, or ~2 x 103 s-1 in methanol. We theorize that E-3a is more flexible than E-7 and therefore it undergoes lactonization more rapidly.
Thus, 1a can be used as a PRPG for alcohols. In comparison, photolysis of 1b yield Z-3b. However, Z-3b does not the release the methanol moiety but rather re-generates 1b. Consequently, 1a has potential as a photoprotecting group for alcohols in applications, whereas 1b can be used for control experiments, as it has similar structure and photochemistry as 1a but does not release its alcohol moiety.
The major difference between benzophenone and butyrophenone based PRPGs 5 and 1 is flexibility. The increased flexibility allows E-3a to release its alcohol moiety faster than E-7. However, with increased flexibility rotation barrier become important. The increased flexibility of 1b compared to 5, prevents it from releasing it alcohol moiety because rotation prevents 2b from forming E-3b. Thus, as a general conclusion for designing PRPGs based on photoenolization and lactonization, is that increased flexibility can make the lactonization faster, but potential rotation barrier can allow other process to compete with the photorelease.
Scheme 3.
This project was done by my graduate students Siva Muthukrishnan and Jagadis Sankaranarayanan and two undergraduate students Mariel Demichiei and Michael Meese. Some of the transient spectroscopy was performed at the University of Victoria in collaboration with Professor Cornelia Bohne and her graduate student Tamara Pace. We have submitted a manuscript entitled The Effect of Alkyl Substituents on Photorelease from Butyrophenone Derivatives. to the J. Org. Chem. based on this work.
2. My graduate students Siva, Jagadis and I wrote a chapter on PRPGs for Adv. Phys. Org. Chem. This chapter focuses mainly on PRPGs that rely on photoinduced intramolecular H-atom abstraction to form photoenols that then release their protected or caged molecules. However, we also compared these photoremovable protecting groups to others that release their protected molecule by different mechanisms. The examples in the chapter did not offer a comprehensive review of the literature but rather illustrated the mechanisms of photorelease from several photoremovable protecting groups, thus making it easier to determine their ability and limitations in distinct applications.
The Chapter is entitled Photoremovable Protecting Groups Based on Photoenolization and it has been published in Adv. Phys. Org. Chem. 2009, Vol. 43, Chapter 2, p 39. We gratefully acknowledged the support of the PRF of our work.
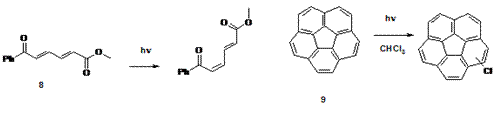
3. This summer two undergraduate students Samantha Yakey (Wheeling Jesuit University, NSF-REU) and Chester Williamson (Central State University, NSF-REU) worked on synthesizing 8 and 9 and study their photochemistry. They used product studies, laser flash photolysis and molecular modeling to determine their photoreactivity. Currently, Qian Li is expanding these research projects and synthesizing derivatives of molecules 8 and 9 which can serve as phototriggers.
Scheme 4.