Reports: AC7
46082-AC7 Basic Mechanisms Responsible for Morphology Control within Polymer Nanoparticles
Understanding morphology control for waterborne, multiphase, polymer nanoparticles is an ongoing endeavor. Upon consideration of composite nanoparticles produced by emulsion polymerization, one must ask how do the different polymer phases come to reside where they do within a particle? Many scholars currently accept an anchoring mechanism in which initiator end groups remain at particle surfaces. This work has developed a series of latex polymerizations which provide evidence against anchoring. One such latex (BSP3-49) is composed of a p(styrene-co-butyl acrylate) seed (dry Tg ~57oC) and a p(MMA) second stage (dry Tg ~ 120oC). The p(MMA) was polymerized with potassium persulfate initiator (KPS) to impart charged SO4- end groups to the polymer chains, and the seed was polymerized with a non-ionic initiator (VA-086) that contains no sulfur.
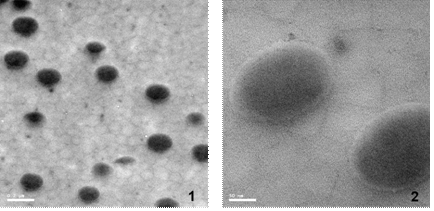
Reaction temperature (TR) was 70oC, and in a semi-continuous (monomer starved) polymerization, particles were produced which contained a core of mixed first and second stage polymers and a thin shell of p(MMA) (transmission electron micrograph, TEM, displayed in Figure 1). The styrene units of the seed were stained with ruthenium tetraoxide (RuO4) for contrast.
Figure 1: TEM micrographs of RuO4 stained sections of BSP3-49 (50-70 nm thickness). 1.) 10,000x and 200 nm size bar. 2.) 40,000x and 50 nm size bar.
The dark phase material was determined be a mixture of p(MMA) and p(St-co-BA) by observation of differential scanning calorimetry (DSC) data for this sample.
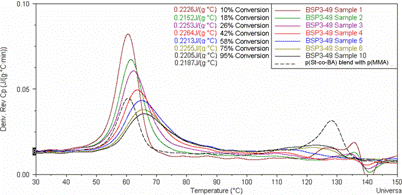
Figure 2: DSC curves of various samples of BSP3-49 at different monomer conversions. The DSC trace of a blend of p(St-co-BA) (dry Tg ~ 60oC) and p(MMA) latices is included for reference.
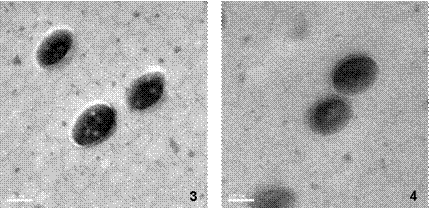
The DSC curves indicate mixing because of both the positive temperature axis shift and negative Cp axis shift as monomer conversion increases. Pure phase polymers should exhibit Tg values at their respective Tg, whereas mixed phase polymers exhibit Tg values between those of the pure phase polymers from which they are made. As another piece of evidence for phase mixing, wet latex samples of BSP3-49 were thermally annealed at 150oC in order to allow the particles to approach their equilibrium conformations. TEM images of these samples are shown in Figure 3.
Figure 3: TEM micrographs of RuO4 stained sections of BSP3-49 (50-70 nm thickness) which were annealed for various times. 1.) 1 hour annealed, 20,000x. 2.) 16 hours annealed 20,000x. Size bar is 100 nm.
Clearly, phase separation has occurred as a result of annealing, since occlusions of p(MMA) become visible.
In addition to these data, SO4- end group titration reveals that while there are SO4- groups detected on particle surfaces, much less than 50% of the total SO4- groups added to the particles are on the surfaces of the particles. It should also be noted that the number-average molecular weight (Mn) of the p(MMA) was determined by gel permeation chromatography (GPC) to be ~64,000 g/mol, which is well below the limit for chain transfer to monomer effects (106 g/mol). It was desirable to eliminate chain transfer to monomer because it may cause unrestricted entry and diffusion of polymer chains in latex particles.
The data presented provide strong evidence against the case of predominant chain end anchoring. Inward chain diffusion becomes more clear upon examining the polymerization history of each particle. At early stages of the monomer starved polymerization, the effective Tg (Tgeff) of the particles is less than TR, which allows short-chained radicals to diffuse easily into the seed polymer. As more MMA becomes converted to p(MMA), Tgeff of the particle increases, since the Tg of p(MMA) is 120oC and the Tg of the seed is 57oC. Eventually Tgeff becomes equal to TR, which reduces the diffusivity of the growing polymer chains since particle viscosity is increasing. Monomer is still able to diffuse easily through the particle to propagate growing chains, but the already formed large polymer chains become frozen in the p(St-co-BA) matrix and therefore remain mixed. By the latter stages of polymerization, Tgeff is greater than TR and a shell of p(MMA) forms around the kinetically frozen mixed phase polymer. Later, thermal annealing of these particles softens this polymer, allowing p(MMA) chains to relax and phase separate into domains.
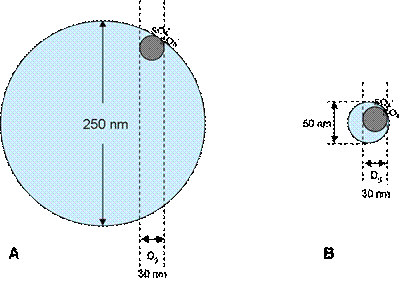
To complement the data and explanations provided for BSP3-49, consider the possibility of p(MMA) chains anchoring to the surface of these ~250 nm particles. If the end groups are anchored, the rest of the chain must be stretched through the particle to fill in the interior domains of p(MMA). The Mn of the p(MMA) chains is large enough to stretch across a 250 nm particle, however there is no apparent driving force for chains to propagate in this manner since propagation occurs in a random walk fashion. Secondly, if chains do anchor, they still must undergo bimolecular termination. This would require the chains to diffuse along a particle surface, which is unlikely since a large polymer molecule would find it difficult to move when entangled with chains of the seed polymer. Finally, the natural state dimensions of a p(MMA) chain would not be large enough to reach the interior of a 250 nm particle, as shown in Figure 4.
Figure 4: Scaled depiction of a surface-anchored second stage polymer chain of Dg = 30 nm in comparison to A.) 250 nm seed particle and B.) 50 nm seed particle. The depiction of the SO4- end groups are not to scale.
Relaxed polymer chains occupy a sphere with a diameter of gyration (Dg). The p(MMA) polymerized for BSP3-49 had a Dg of about 30 nm (as depicted in Figure 4), which is clearly not large enough for the second stage polymer to reside in the far interior of the particle.
The experiment presented portrays one set of data which support the notion that chain end anchoring is not a predominant mechanism for morphological development of composite nanoparticles. As a graduate student who executed these experiments and analyses for a dissertation, I have evolved a deep understanding of the critical need for the underlying comprehension of the theories, concepts, and mechanisms behind any process which is inherently scientific.