Reports: GB1
48347-GB1 New Synthetic Methodology for Ring Formation
Statement of Progress and Significance
In the past year, the time, effort, and resources have been devoted to both projects described in the original PRF proposal (the synthesis of schischkiniin and the synthesis of cyclobutanones and lactones via nucleophilic catalysis). This PRF grant award allowed several students to participate successfully in undergraduate research projects, both as independent studies for credit and as paid fellowships over the summer. The efforts of the students combined with my own investigation resulted in significant progress on both of the proposed projects and paved the way to a new avenue of research. The successes and failures of the experiments provided a unique and irreplaceable learning experience for the students for which we are deeply grateful.
I. Enantioselective Nucleophilic Catalysis Synthesis of Cyclobutanones and γ-Lactones
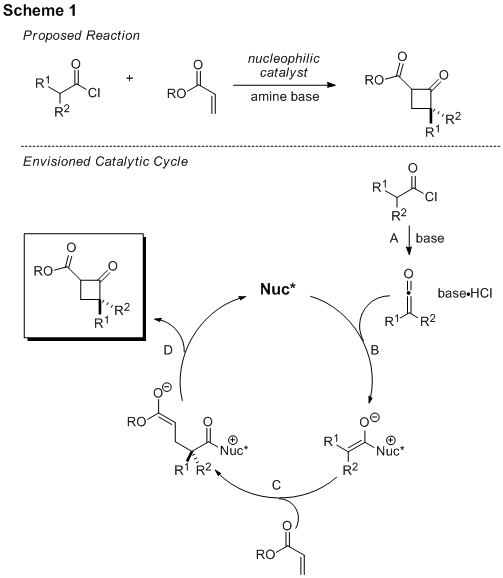
During the summer of 2009, a detailed investigation of the proposed nucleophile-catalyzed reaction to produce cyclobutanones (Scheme 1) was undertaken.
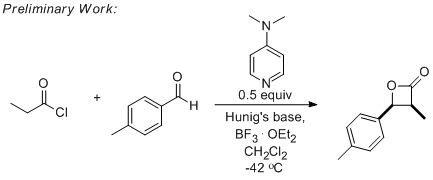
Initial experiments were aimed at optimizing a known process to master ketene and ketene-enolate generation as well as to observe nucleophilic catalysis before trying to develop the new reaction. Several experiments were performed with 4-dimethylaminopyridine as nucleophilic catalyst with two different acid chlorides and 4-methylbenzaldehyde. It was found that a substituted ketene, formed from propionyl chloride, is more reactive than ketene itself, formed from acetyl chloride. Additionally, we found that a Lewis acid facilitates the desired process when using dimethylaminopyridine as a catalyst. Low temperatures (-42 and -78 °C) promote the desired process more effectively than higher temperatures (0 and 25 °C). While yields in our hands were low to moderate, this initial study set the stage for application of the catalyst system to a new reaction.
Next, the formation of a cyclobutanone was attempted. Given our preliminary results, propionyl chloride was selected as the ketene precursor with DMAP as the nucleophilic catalyst. We began by using t-butyl acrylate as the α,β-unsaturated ester. Several temperatures and reaction conditions were screened, but the only new product identified was a polymer of propionyl chloride. The formation of this product suggests that the α,β-unsaturated ester is not electrophilic enough to react with the ketene-enolate. Our hypothesis led us to choose an α,β-unsaturated ester with a strong electron-withdrawing group attached, 2,2,2-trifluoroethyl acrylate, as the coupling partner. This more reactive acrylate partner appeared to produce new products when exposed to the reaction conditions, however these products are unstable to the work-up procedure. Our future investigation will focus on other electrophilic acrylate coupling partners as well as additional catalysts (including the cinchona alkaloids) and Lewis acids.
II. New Methods for Total Synthesis Macrocyclization via NN Bond Formation and [2+2] Cycloaddition.
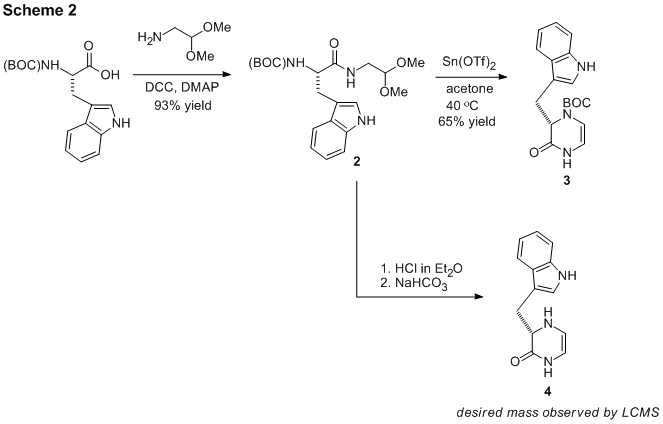
Work on the synthesis of schischkiniin (1) to probe a new macrocyclization method was also begun. As shown in Scheme 2, Boc-protected tryptophan was successfully coupled with 2,2-dimethoxyethylamine to produce dipeptide 2 in excellent yield. This compound smoothly underwent a Lewis acid-catalyzed sequence involving 3 steps (deprotection, cyclization, and dehydration) to furnish the key intermediate dihydropyrazinone 3. A related and more complicated sequence involving an additional deprotection step was attempted using protic acid catalysis, but the product(s) of the reaction was largely insoluble in organic solvent and difficult to characterize. However, LCMS analysis of the product mixture showed a major product with the mass of the desired dihydropyrazinone 4. Future investigation will be devoted to product isolation and characterization.
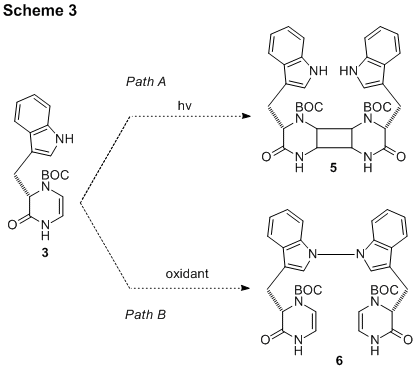
With purified compound 3 in hand, the path was cleared for the investigation of the requisite cycloaddition and N-N bond formation oxidation reactions (Scheme 3). First, the UV absorption spectrum of 3 was obtained to select the appropriate wavelength of light for photocycloaddition reactions. To our disappointment, very little reaction occurred when 3 was irradiated for several days (Path A). The addition of a catalyst, copper triflate, did not improve the reactivity and the desired compound 5 has not been isolated yet. It is suspected that compound 3 is sterically hindered due to the large BOC protecting group. Future removal of the BOC group is planned and the cycloaddition will be studied using compound 4.
The alternate order of synthetic steps was also investigated (Path B). That is, compound 3 was exposed to oxidizing agents to attempt dimerization via N-N bond formation. It was found that treatment with ceric ammonium nitrate caused immediate decomposition of 3, while exposure to sodium persulfate did not cause any reaction to occur. A simpler indole-containing compound was studied to simplify the problem. To date, the needed dimerization process has been elusive using chemical oxidants; compound 6 has not been isolated yet. The next direction of research will be to study electrochemical oxidative methods.
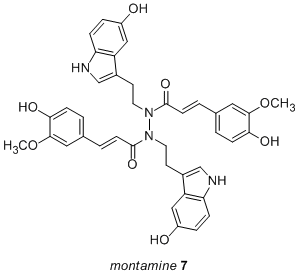
III. New Directions Schischkiniin Inspires a Bidirectional Approach to the Synthesis of Montamine
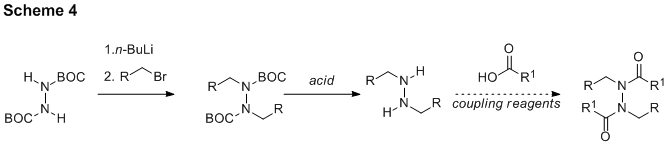
The same research group that isolated schischkiniin recently reported the isolation and characterization of a new, related natural product target: montamine (7). It is related to schischkiniin by its symmetry and central N-N bond. While synthesis via N-N bond formation from two equivalents of a simple amide seems ideal, a method to effect this transformation does not currently exist. An alternative approach exploiting the power of symmetry and bidirectional synthesis has been identified (Scheme 4). We have undertaken introductory experiments to probe the feasibility of this approach, and we have successfully achieved a model double alkylation-double BOC removal sequence. We plan to investigate the third step of the route, the double amidation, soon. A synthesis of montamine or derivatives would enable the study of N-N bonds and shed light on the system present in schischkiniin.