Reports: AC10
44674-AC10 Three-Dimensional Reconstruction of Mesoporous Materials from Gas Adsorption and Structure Factor Data
Over the term of this project we have developed new theoretical and computational methods for the prediction of gas adsorption in porous materials. Specifically, we have developed a new density functional theory of high-resolution lattice fluids, which we have shown is quantitatively accurate for adsorption in microporous and mesoporous materials at only modest computational cost. This theory bridges between existing continuum density functional theories and lattice-gas calculations, providing the precision of the former with the convenience of the latter. Unlike previous methods, this approach can be used for the prediction of adsorption in microporous materials such as zeolites, carbon nanomaterials and coordination polymers such as metal-organic frameworks (MOFs), coarse-grained models. Adsorption in such materials is of significant industrial importance, impacting catalysis and separations. Adsorption in microporous materials is also frequently suggested as the basis for storage of hydrogen and methane gases.
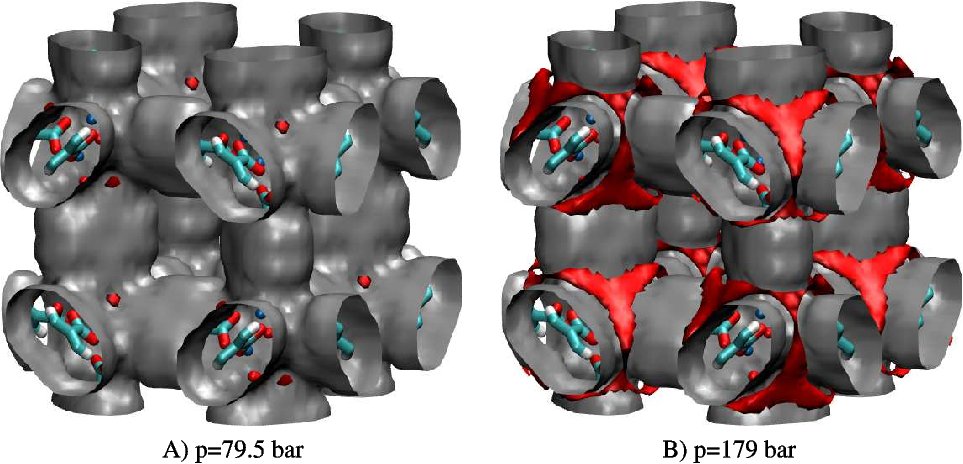
Our theory is the first computationally tractable adsorption model that can be applied in realistic models of microporous materials; all previous adsorption modeling using fluid density functional theory have relied on idealized models and high symmetry to reduce the dimensionality and computational cost of the problem. The method is based on an lattice re-derivation of Tarazona's (1985) original continuum weighted-density (off-lattice) theory, with appropriate parametrization of hard-sphere terms against simulation results for the hard-sphere lattice fluid. Because the lattice approximation is made at the level of the underlying model rather than the integration, the usual integrals are replaced by exact summations and numerical difficulties are greatly reduced. As an example application, Figure 1 shows surfaces of constant adsorbed hydrogen density in the MOF-5 material.
Figure
1. Hydrogen adsorption in MOF-5 at 298 K, at two different pressures. Gray isosurfaces correspond to a local density of 1.33 mol/l and red
isosurfaces correspond to a local density of 23.2 mol/l. The red
isosurfaces clearly identify the adsorption sites near the zinc
atoms.
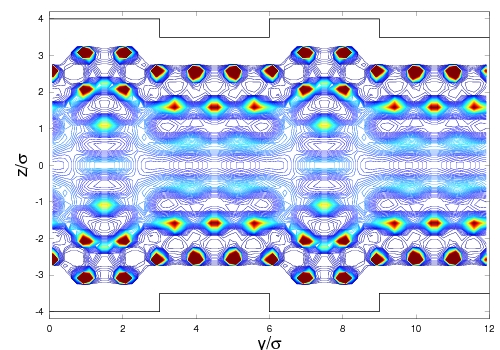
Work in the third year of the project has focused on further improvements of the lattice theory and its application in fundamental studies of gas adsorption in complex pore geometries. In the former area, we have completed an exhaustive study of the properties of the lattice hard-sphere fluid, necessary for optimal choice of lattice spacing and other parameters. As an example of our work on fundamentals of capillary phenomena, Figure 2 displays density contours of gas adsorbed in a "crenellated" pore. Using the new density functional theory approach it is clearly possible to resolve the complex behavior of adsorbed gases in structured materials. Calculations such as this will in the future contribute to the rational design of materials for specific adsorption applications in energy-related areas such as gas separations and purification.
Figure
2. Density contours of nitrogen multilayer adsorption in a
slit-shaped 2.89 nm width carbon pore with "crenellated"
walls. The wall corrugation pitch is 1.05 nm in width and only 0.175
nm in height. It nonetheless breaks translational symmetry along the
pore wall, leading to sharply localized adsorption in the first and
second adsorbed layers, with weaker effects visible in the center of
the pore.




