Reports: DNI10
49007-DNI10 Electroceramic Materials for High-Purity Hydrogen Extraction from Liquid Hydrocarbon Fuels: Fundamental Investigations of Coupled Electrochemical and Catalytic Phenomena
Summary
Cobalt-doped barium cerate-zirconate perovskites (BaCoxCeyZr1-x-yO3-a) displaying both catalytic and mixed-conducting properties for high-purity hydrogen extraction from methanol have been synthesized and analyzed to verify crystal phase, structure, surface morphology and composition. Catalytic activity of resulting electroceramic for oxidative reforming of methanol has been investigated, and on-going impedance-spectroscopy analysis have made strides in quantifying both ionic and electronic conductivities of the material. These findings support continued investigations of coupled catalytic hydrogen production and electrochemical purification using these novel materials.
Material Synthesis
BCZC was synthesized via the route of oxalate co-precipitation, using metal nitrate precursors and oxalic acid precipitant at 50oC and pH 2 for 2.5 h. Aqueous NH4OH solution was used to set pH of the reaction mixture. The pink precipitate was filtered out and washed with de-ionized H2O followed by 0.5 L C2H5OH, and then dried at 140oC for 3 h. Powder was then calcined at 1550oC in air for 4 hours on a Pt foil placed in an Al2O3 crucible, with heating and cooling rates of 5oC/min.
Materials Analysis
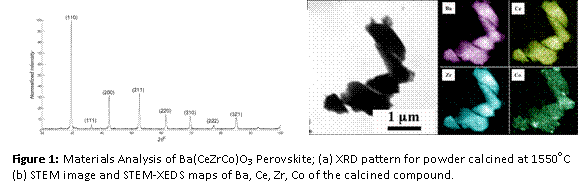
X-ray diffraction (XRD), transmission and scanning transmission electron microscopy (TEM & STEM) coupled with X-ray energy dispersive spectroscopy (XEDS) were used to verify successful fabrication of target homogeneous material. Powders were characterized by XRD (Bruker Axs D5005 diffractometer, Cu-Kα radiation) over the 2θ range of 20 100o at a scan rate of 2o/min. An XRD pattern is presented in Figure 1a. The pattern could be indexed with a high degree of accuracy to a single cubic phase with a = ~4.28 Å.
Values of d-spacing measured from the electron diffraction pattern from STEM analysis match closely with the XRD result. The dimensions of the crystalline particles after sintering at 1550oC are micrometer in size. In order to determine the chemistry of the particles, STEM-XEDS analytical mapping was carried out on several particles. The image of a particle and the corresponding analytical maps for Ba, Ce, Zr and Co are given in Figure 1b. The elements are homogeneously distributed without any signature of localized segregation up to nanometer length scale.
Catalytic Investigations
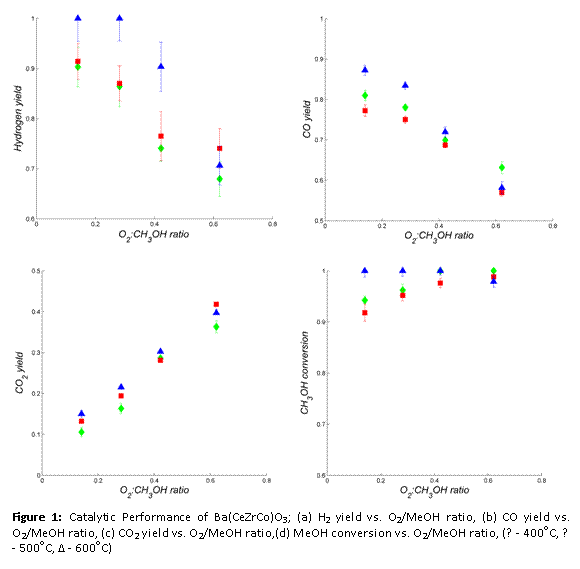
BCZC was tested for catalytic activity towards hydrogen generation via partial-oxidation of CH3OH at different temperatures and O2:CH3OH ratios, with reaction products analyzed on dry basis (after dehumidification in a dry-ice moisture trap) using a GC. Hydrogen, CO, CO2 and CH4 were detected in the product mixture. N2 was used as an internal standard for calculating outlet flows of products. Results of reforming experiments are presented in Figure 2. Hydrogen yields of greater than 60 % were obtained for each of the test conditions, with CH3OH conversions from 90 % to 100 %. These results indicate that BCZC is a potential catalyst for hydrogen generation by CH3OH partial-oxidation.
Electrochemical Impedance Spectroscopy (EIS)
Impedance spectroscopy tests (Solartron potentiostat SI 1287, Solartron frequency response analyzer SI 1260) were conducted on BCZC pellets in different atmospheres at different temperatures with both Pt and Ag electrodes. As discussed below, protonic conduction can occur either by interaction of hydrogen with lattice oxygen or by interaction of H2O with oxygen vacancies. Electronic conductivity was determined by impedance spectroscopy in dry, inert atmospheres to eliminate ionic charge carriers. Hence, dry N2/He, wet N2/He and wet N2/He/hydrogen were selected as atmospheres for the impedance spectroscopy measurements. All tests were conducted in the frequency range 1 MHz to 0.1 Hz with an ac amplitude of 10 mV. Cylindrical pellets (13 mm die) were made by cold-pressing BCZC powder at approximately 210 MPa, followed by sintering at 1550oC. Electrodes consisted of a flat circular Pt spirals spot-welded to a Pt mesh (52 mesh, woven from 0.1 mm diameter wire). The BCZC pellet was sandwiched between two such mesh/spiral electrodes inside a quartz tube-in-tube set-up with provision to maintain desired atmosphere around the pellet. Platinum wires (0.5 mm diameter) were used as current collectors.
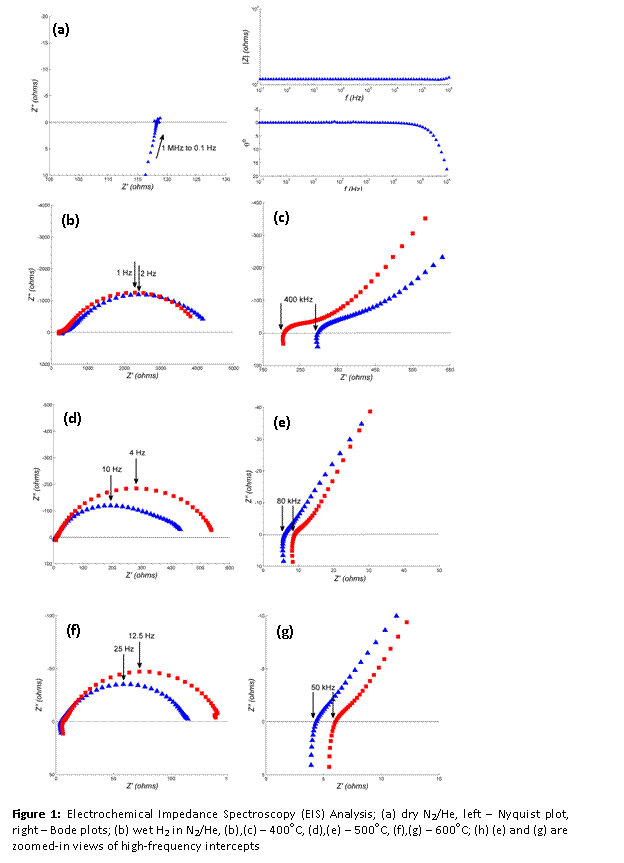
Impedance spectroscopy was conducted on samples under different temperature, atmosphere & electrode-material conditions and results were recorded as Nyquist plots. Representative plots under dry N2/He are presented in Figure 2. Plots under dry N2/He were similar in shape irrespective of temperature or electrode material. These plots ended in a low-frequency real-axis intercept (Z′ @ 0.1 Hz), which represents a pure resistive component. Due to the absence of any other feature on the Nyquist plot, this intercept was attributed to the bulk resistance of the electrolyte. Conductivity values calculated exhibited Arrhenius type dependence with respect to temperature. Electronic conductivity in BCZC under dry N2/He was estimated as 3.8 mS/cm at 600oC.
Under conditions where the electrolyte acts as an ionic conductor, Nyquist plots obtained with symmetrical cells typically consist of overlapping arcs, with the high-frequency arc attributed to bulk-phase conduction and overlapping low-frequency arc representing grain boundary conduction. Nyquist plots obtained under wet hydrogen are presented in Figure 2. In the presence of hydrogen the following reaction may occur:
Plots suggest that hydrogen introduction contributed significant protonic conductivity to the electrolyte via Reaction I. Since BCZC displays comparable electronic conductivity at lower temperatures and ionic conductivity in the presence of hydrogen, it is a promising candidate for hydrogen permeation by means of mixed protonic-electronic conductivity.
Publications Resulting from Effort:
None to date. Efforts are underway to publish materials analysis in the Journal of Materials Science, while electrochemical analysis is being prepared for publication in the Journal of the Electrochemical Society.