Reports: G10
46793-G10 Surface-Initiated n-Type Semiconducting Polymer Synthesis
The overarching goal of the ACS PRF Type G Award has been create a surface-grafted semiconducting polymer with the view of obtaining an additional handle of controlling polymer morphology and orientation that could lead to new properties in optoelectronic devices, which include improved charge mobility within the polymer film. The first year of the proposal focused on a step-wise procedure to create the surface-grafted polymer. In the second year, we have moved our focus to a more efficient way to create a surface grafted polymer using a one-step living polymerization procedure. Further details for developing a living polymerization procedure are described below.
The ability of chemists to design and synthesize p-conjugated organic polymers with precise control over the molecular weight with narrow polydispersities remains the key to technological breakthroughs using polymeric materials in electronic and photonic devices. Being able to synthesize semiconducting polymers with control over the molecular weight with narrow polydispersities will reduce the variability between synthetic runs which can lead to differences in electronic device performances between different research labs. Additionally, being able to synthesize well-defined block copolymers, star shaped polymers and surface grafted polymers will expand the structural library of semiconducting polymers which will allow us to obtain a more in-depth knowledge of the relationship between the structure and electronic properties of semiconducting polymers. In order to realize the goal of controlled polymerization and to be able to create additional polymer architectures, a new polymerization technique must be utilized. Regioregular poly(3-hexylthiophene) (rr-P3HT) has received much attention in photovoltaic application in recent years because of its small band gap, high hole mobility, and good solubility. Regiocontrolled synthesis of poly(3-alkylthiophenes) has been developed by McCullough and Rieke, and the recent discovery of chain-growth polymerization of rr-P3HT that was reported by McCullough and Yokozawa has attracted our attention as a gateway to achieve our goals. Polythiophene containing block copolymers, as well as conjugated polymers such as poly(p-phenylene)s and polypyrroles have been successfully synthesized by a handful of researchers using the Kumada catalyst-transfer polycondensation (KCTP), also commonly known as the GRIM method. The chain-growth polymerization of polyfluorenes, which utilizes a Suzuki coupling, has also been investigated.
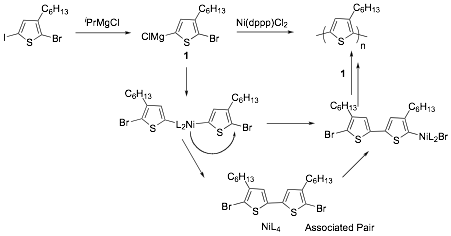
Scheme 1. Polymerization Mechanism according to McCullough and Yokozawa.
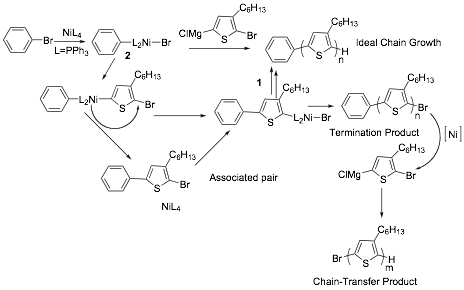
The mechanism for the KCTP was proposed by McCullough and Yokozawa as shown in Scheme 1. According to the reaction scheme, the 5,5'-dibromo-4,4'-dihexyl-2,2'-bithiophene chain initiator is generated in-situ via transmetallation of two molecules of 1 at their magnesium ends with Ni(II)dpppCl2 yielding the symmetrical bis-organonickel compound, which is thought to be the initiator of the polymerization. In the next step, the Ni either intramolecularly transfers to the C-Br bond end of the polymer or reductively eliminates but re-inserts into the C-Br bond of the same growing polymer chain without diffusion into the surrounding solution. Thus based on this unexpected ability of Ni catalyst to migrate intramolecularly, polymerization occurs with addition of one monomer unit at a time leading to a chain growth polymerization mechanism. In this mechanism, one Ni molecule forms one polymer chain, so the molecular weight of the polymer is proportional to Ni loading and the polydispersity index is very narrow. Kiriy et al. and Locklin et al. have succeeded in applying KCTP in the synthesis of poly(3-hexylthiophene) brushes on the surface. However due to chain-transfer mechanisms, if the polymer is grown from a monolayer, the brush film thickness is low. The goal of our work was to develop an alternative initiation strategy that would provide a polymer with greater initiation efficiency.
Scheme 2. Catalyst-transfer polymerization from small molecule initiators according to Kiriy et al.
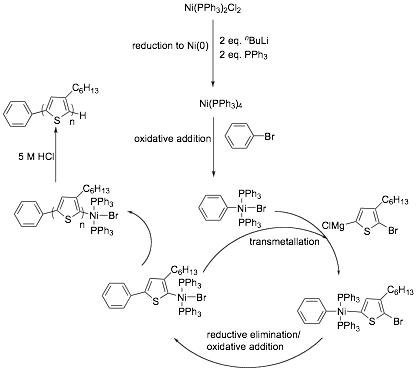
We have now developed a more facile method (Scheme 3) for the externally initiated growth of rr-P3HT from various aryl halide initiators, which provides rr-P3HT with enhanced initiation efficiencies, greater regioregularity and increased molecular weights. The investigation into the relationship between the type functional group on the initiator and the polymerization mechanism was also performed.
Scheme 3. Catalytic Cycle of Ni(PPh3)2Cl2.
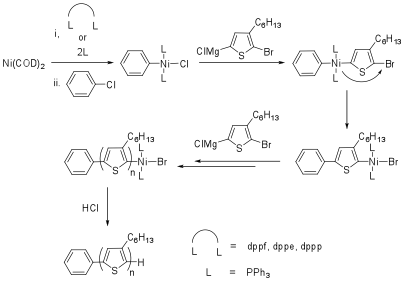
In the above, although our method of initiation provided polymers with improved regioregularity and PDI compared to previous methods, we were still restricted to using PPh3 as a ligand and were unable to perform the initiation using dppp as the ligand, which provides the best control for P3HT polymerizations. Additional drawback of these systems is the inability to easily vary the structure of the spectator ligand, thus preventing a full investigation into factors which may improve externally initiated polymerization control. In order to obtain a controlled polymerization for an externally initiated polymerization, it would be advantageous to use a bidentate ligand such as 1,3-bis(diphenylphosphino)propane (dppp) as the catalyst chelate, as these have been shown to have optimal bite angles (~90o) which results in the desired relative rates of oxidative addition, transmetallation and reductive elimination in the catalytic cycle, providing polymers with a narrow PDI, controlled molecular weight, and high regioregularity.With this in mind, we investigated a synthetic methodology for the external initiation of P3HT whereby the catalyst system can be systematically varied allowing investigation into how it affects the outcome of the polymerization. The work was performed in order to increase our understanding of the role of the ligands on the external initiation for the synthesis of P3HT, and to try and understand why the much needed use of dppp has failed so far for the external initiation. Previous initiation methods cannot be used because they only allow the use of PPh3 as a ligand. The use of Ni(COD)2 allows us to introduce the ligand of choice (Scheme 4). The four ligands used in this study were triphenylphosphine (PPh3), 1-1'-bis(diphenylphosphino)ferrocene (dppf), 1,2-bis(diphenylphosphino)ethane (dppe) and 1,3-bis(diphenylphosphino)propane (dppp) and were chosen due to their extensive use in metal catalyzed carbon-carbon bond forming chemistry. A preliminary study on the effect of regioisomerism on the initiating species has also been performed. The award resulted in two publications and 16 invited presentations during the current award period.
Scheme 4. Synthesis of externally initiated P3HT