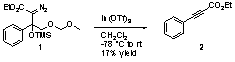Reports: G1
47627-G1 Concise and efficient routes to polycyclic nitrogen heterocycles by way of a novel ring fragmentation reaction
The overarching objective of this research is to establish new synthetic organic methodology to prepare functional group rich compounds that will in turn facilitate the preparation of structurally complex nitrogen or oxygen containing polycyclic heterocycles. The generous financial support provided by the ACS- PRF has allowed us to develop a novel ring fragmentation reaction that in turn provides an efficient 3-step sequence to prepare polycyclic 2,5-dihydropyrroles from simple α-silyloxyketones. Efficiency is an increasingly important consideration in the strategic planning of synthetic sequences in part because of the important role that environmental considerations have begun to play in the field of synthetic chemistry, and yet many synthetic sequences still depend on small iterative structural changes. To address the issue of efficiency in synthesis our work focuses on developing reactions that result in big structural changes in molecular architecture. By making big structural changes in one-step we hope to render synthetic routes more efficient than if only iterative transformations were used. Details of work supported by this grant have been reported in a JACS communication as well as a Ph.D. dissertation. A second manuscript has been submitted to JOC, and a third manuscript is in preparation and will be submitted to JOC in the near future.
This research stems from our attempts to prepare heterocycles from diazo compounds. To this end we prepared α-diazoester 1 (Scheme 1) as a potential heterocycle precursor. When we treated diazo 1 with Indium(III) triflate we did not obtain the desired heterocyclic product, but instead obtained a complex mixture from which we isolated ethyl 3-phenylpropiolate (2) in 17% yield. The formation of propiolate 2 appeared to involve loss of the β-silyloxy group, loss of molecular nitrogen and fragmentation of the Cβ-Cγ bond.
Scheme 1
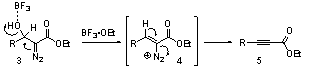
A search of the literature revealed that Wenkert and McPherson had reported that β-hydroxy-α-diazo esters (e.g. 3, Scheme 2) also react with Lewis acids to provide ynoates by the mechanism shown in Scheme 2.
Scheme 2
Based on this precedence, we considered it likely that our observed reaction occurred as shown in Scheme 3. Lewis acid induced elimination of the β-silyloxy group would provide vinyl diazonium intermediate 7, which would undergo a Grob-type fragmentation in which electron donation from the γ-oxygen and loss of molecular nitrogen would provide the alkyne product.
Scheme 3
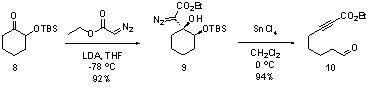
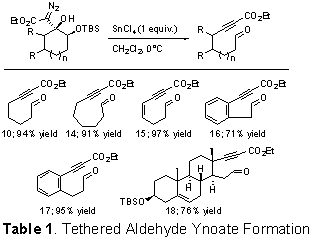
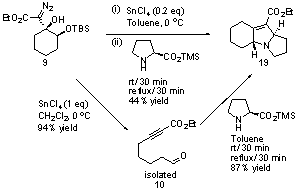
The fragmentation of the Cβ-Cγ bond in the above system intrigued us and we hypothesized that cyclic γ-oxy-β-hydroxy-α-diazo esters (e.g. 9, Scheme 4) in which the Cβ-Cγ bond was contained within a ring, would undergo a Lewis acid-mediated ring fragmentation to provide tethered aldehyde ynoates (e.g. 10). In order to promote the desired fragmentation step it seemed logical to make the γ-oxygen more electron rich and to test our hypothesis we prepared α-diazo ester 9 (Scheme 4) as a model system. Diazo ester 9 is trivial to prepare by simply adding LDA to a pre-mixed solution of α-silyloxy ketone 8 and commercially available ethyl diazoacetate.
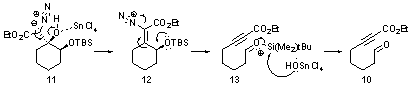
Consistent with our hypothesis, treating diazo 9 with tin tetrachloride resulted in vigorous gas evolution and provided aldehyde tethered ynoate 10 in 94% yield. Our current mechanistic hypothesis for this transformation is shown in Scheme 5. Reducing the quantity of tin tetrachloride to 10 mol% successfully promoted the fragmentation reaction, but reduced the product yield to 85%. Changing the solvent from CH2Cl2 to toluene had little effect on the reaction, whereas DMF inhibited the reaction completely.
Scheme 4
Scheme 5
This novel ring fragmentation is an important discovery because there are few synthetically useful fragmentation reactions known, and none that provide the aldehyde ynoate functional group combination. The fragmentation reactions that are known hold a special position in organic synthesis because they can unmask latent functional groups under chemoselective reaction conditions and they can provide functionalized synthetic intermediates that are otherwise difficult to prepare.
We have conducted an exploration of the substrate scope of this fragmentation and it appears to be fairly general. These results are summarized in Table 1 and have been published as a communication in the Journal of the American Chemical Society acknowledging support from the ACS-PRF.
More recently we have discovered that this fragmentation is also applicable to the preparation of tethered aldehyde ynones by fragmenting substrates derived from the LDA mediated condensation of diazo ketones with α-silyloxy cyclohexanone. The fragmentation again appears to be fairly general and alkyl and aryl ynones are formed in good yield. Of particular note, diazo ketones derived from amino acids also readily fragment to the corresponding α-amino ynone products with retention of the α-stereocenter. These results will be submitted as a full paper acknowledging ACS-PRF support to the Journal of Organic Chemistry in the near future.
In terms of achieving our overarching objective of establishing new synthetic organic methodology that will facilitate the synthesis of complex heterocycles, we have recently begun to exploit the above fragmentation products in intramolecular reactions. Specifically, we have identified that polycyclic 2,5-dihydropyrroles, a heterocycle framework found in many useful natural products, can be efficiently prepared from the above tethered aldehyde ynoate fragmentation products by an intramolecular 1,3-dipolar cycloaddition. For example, diazo 9 was converted to tricyclic 2,5-dihydropyrrole 19 (Scheme 6) in 44% yield by a one-pot fragmentation / azomethine ylide 1,3-dipolar cycloaddition sequence. The yield of 2,5-dihydropyrrole 19 improves to 83% over two steps when the fragmentation product is isolated prior to dipolar cycloaddition. This sequence of reactions is illustrative of our goal, which is to develop efficient routes to structurally complex heterocycles from simple precursors.
Scheme 6
With these preliminary results in hand, we plan to develop the fragmentation / 1,3-dipolar cycloaddition sequence to prepare more structurally complex heterocyclic systems. Overall, this research is providing novel ways to efficiently prepare synthetically challenging structures from readily available starting materials.