Reports: G6
45787-G6 The Ignition and Oxidation of Potential Oxygenated Fuel Additives
Summary
As part of this ACS-PRF funded project a new shock tube has been designed, constructed, characterized, and validated for high-temperature combustion chemistry experiments and the high-temperature ignition and oxidation of several potential oxygenated octane-boosting fuel additives has been investigated. These oxygenated compounds include the four butanol isomers and 2,5-dimethylfuran. Shock tube experiments were performed to characterize the differences in the high-temperature reactivity of these potential fuel additives. Additionally, kinetic modeling for the four butanol isomers elucidated the importance of the various classes of consumption reactions (dehydration, unimolecular decomposition, and H-atom abstraction) for the four butanol isomers and provided predictive modeling of the observed ignition phenomena.
New Shock Tube
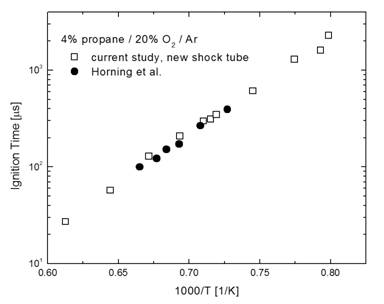
A new fully instrumented high-purity shock tube (12.3 cm inner diameter and 10.6 m total length) has been designed and constructed for the high-temperature investigation of combustion chemistry (Figure 1). The shock tube has been fully characterized and validated. Validation experiments included the measurement of ignition delay times for propane/oxygen/argon mixtures for comparison to the measurements of Horning et al. (2002), which we have a high level of confidence in; see Figure 2 for a comparison.
Figure 1. The new RPI shock tube.
Figure 2. Comparison of the previous Horning et al. (2002) propane shock tube ignition measurements to validation measurements made in the new RPI shock tube.
Oxygenated Fuel Additive Ignition Studies
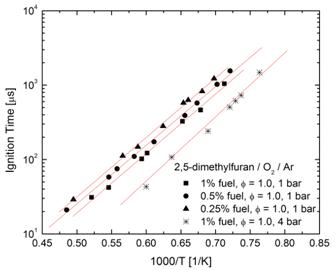
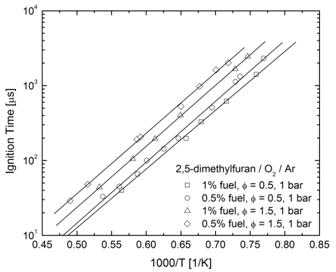
The four butanol isomers (1-, 2-, iso-, and tert-butanol) and 2,5-dimethylfuran have received interest as potential high-octane rated oxygenated additives for gasoline. However, the literature contains little information about the high-temperature oxidation and ignition characteristics and kinetics for these compounds, which is of interest if these compounds are to find future implementation as fuel additives for internal combustion engines. Hence, the autoignition of these compounds has been experimentally studied at high temperatures in the recently constructed shock tube at Rensselaer. Ignition delay times for fuel/oxygen/argon mixtures have been measured behind reflected shock waves using electronically excited OH emission and pressure measurements to determine ignition delay times for the five oxygenated compounds of interest; see Figure 3 for an example ignition measurement for 1-butanol. Measurements were made for a variety of fuel, O2, and Ar concentrations (analogous to a variety equivalence ratios, fuel mole fractions, and pressures) and temperatures (1200-1900 K) to determine the dependence of ignition on these parameters. For illustrative purposes the entire data set for 2,5-dimethylfuran is shown in Figure 4; similar data sets were obtained for all compounds studied.
Figure 3. Example butanol ignition delay time measurement.
Figure 4. Ignition measurements for 2,5-dimethylfuran.
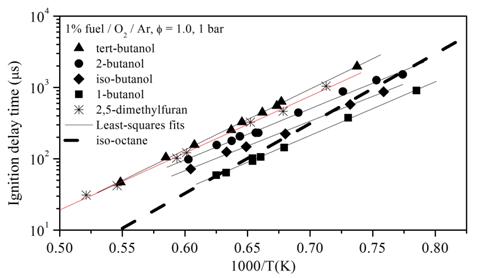
The ignition times for all the oxygenated compounds show similar trends but exhibit large differences in the absolute ignition times for a given temperature and mixture composition; see Figure 5. The differences in ignition time, a fundamental indicator of high-temperature reactivity, for the most and least reactive compounds studied, 1-butanol and tert-butanol respectively, spans approximately a factor of five. These large differences in high-temperature reactivity will have significant consequences for the use of these compounds as octane-boosting fuel additives to gasoline. Gasoline representative compounds, such as iso-octane, have ignition times similar to 1-butanol, albeit with greater overall activation energies (slope in Figure 5), under high-temperature conditions; the ignition times for iso-octane under similar conditions are shown in Figure 5.
Figure 5. Ignition comparisons for the studied oxygenates and iso-octane for common conditions.
A detailed kinetic mechanism has also
been developed, in collaboration with Frederique Battin-Leclerc and co-workers
at Nancy University, to describe the oxidation of the four butanol isomers. The
butanol mechanism has been validated through comparison with the shock tube
ignition measurements. Reaction flux and sensitivity analysis performed using
the detailed oxidation mechanism illustrates the relative importance of the
three competing classes of consumption reactions: dehydration, unimolecular
decomposition, and H-atom abstraction. 1-butanol and iso-butanol,
the most reactive isomers, are consumed primarily by H-atom abstraction
resulting in the formation of radicals, the decomposition of which yields
highly reactive branching agents, H-atoms and OH radicals. Conversely, the
consumption of tert-butanol and 2-butanol, the least reactive isomers, takes
place primarily via dehydration, resulting in the formation of alkenes, which
lead to resonance stabilized radicals with very low reactivity. The mechanisms
not only provide a qualitative explanation for the observed differences in butanol
isomer reactivity but also do an excellent job at predicting the measured
quantitative ignition times for all conditions studied. Presently the PI and
students are developing a detailed kinetic mechanism for 2,5-dimethylfuran
oxidation with aims to predict the ignition measurements and provide insight
into the observed low reactivity of this compound.
Impact of Research
This ACS-PRF supported study of the
ignition and oxidation of oxygenated fuel additives represents a step forward
in the understanding of the high-temperature chemistry of oxygenated fuel
additives. To our knowledge, the ignition delay measurements for the butanols
and 2,5-dimethylfuran are the first of their kind. The experimental ignition
data acquired as part of this project will be of significant value as kinetic
targets for the future development and refinement of kinetic mechanisms for
oxygenated hydrocarbons.
In addition to the scientific impact
of the research produced under the ACS-PRF grant, the grant has had an
important impact on the PI and several graduate and undergraduate students. The
grant enabled the PI to develop a shock tube for the study of high-temperature
combustion chemistry, which will have lasting impact as the facility finds continuous
use in the PI's research activities, and enabled the partially support of three
graduate students during their pursuit of MS degrees (two of these students are
currently PhD candidates with the PI) and three undergraduate students (two of
these students are now graduate students with the PI). The importance of the
ACS-PRF support for the PI and students cannot be understated, as it enabled
the PI to start a research program and the students involved to purse research
and education which will hopefully catalyze life-long contributions in their
chosen scientific and engineering careers.