
ACS PRF | ACS
All e-Annual Reports

43246-G1
Development and Applications of New Reactions for Chemoselective Amidations
1. A Novel, Chemoselective Amide Bond Forming Reaction
Chemoselective amide forming ligations are distinctively significant in the production of large peptides or proteins with synthetic modifications.[1,2] In this method, combining two peptide fragments containing functional groups leads to the formation of a single oligopeptide product comprised of a native peptide bond at the ligation site. We have recently documented a novel amide bond forming reaction arising from the chemoselective coupling of a-ketoacids and hydroxylamines under mild conditions, without the need of coupling reagents and requiring no side chain protecting groups (Scheme 1).[3]
![]()
Scheme 1. A new amidation reaction.
2. Cyclic Hydroxylamines for an Iterative Poly-Beta Peptide Synthesis
Based on this recently developed a-ketoacid-hydroxylamine peptide ligation reaction we have developed a new iterative approach to b3–oligopeptides synthesis (Scheme 3).[4] This coupling-reagent free peptide ligation to form a native amide bond proceeds in water, produces no by-products and tolerates fully unprotected side-chains. The necessary cyclic hydroxylamines, isoxazolidines, suitable for iterative b3-oligopeptide synthesis, are readily prepared by adapting Vasella's diastereoselective nitrone cycloaddition with a carbohydrate-derived chiral auxilary.[5] Facile access to this novel isoxazolidine monomers are achieved in one-pot and can be prepared on a multi-gram scale and isolated with > 99 % enantiomeric excess by chromatography or recrystallization (Scheme 3). The sugar auxilary can be removed and recycled generating no waste in the synthesis of this highly desirable building blocks.

Scheme 2. Asymmetric synthesis of isoxazolidine monomers.
In our initial work we employed D-mannose derived auxiliary 13 however, the higher selectivities and the inexpensive cost of the starting material for both enantiomers of gulose-derived auxilaries have allowed its utilization in the preparation of a wide range of isoxazolidine monomers in both enantiomeric forms (Chart 1, next page).
We have demonstrated the efficacy of this chemoselective decarboxylative peptide ligation for iterative peptide synthesis by preparing enantiomerically pure poly-b3-peptides under aqueous conditions without coupling reagents (Scheme 4, next page).
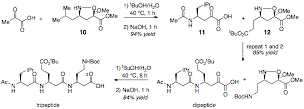
Scheme 3. Iterative synthesis of poly-b3-peptides.
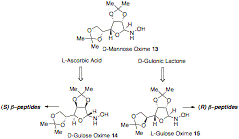
Chart 1. Chiral auxilaries.
In extending our results to practical procedures for the preparation of longer b3-oligopeptides, investigations are currently underway in developing and implementing a solid-phase method for the preparation of b3-oligopeptides that will avoid issues of solubility and hydrophobic collapse of the growing peptide through the use of aqueous conditions and unprotected side chains. Employing our amide ligation methodology, requires a novel linker to allow peptide assembly in an inverse N to C direction on a water compatible resin. The linker should enable assisted amide cleavage for the selective hydrolysis and release of the free N-terminal amide. PL-PEGA resin (Mendal's resin) has proven to have the most ideal properties for the employment of our new amide ligation methodology for iterative b3-oligopeptides synthesis (Sheme 5). Linker 17 has been prepared in seven high yielding synthetic steps.[6] Preliminary results reveal the efficiency of the stepwise couplings on solid support of unprotected sidechain isoxazolidine monomers to the regenerated a-ketoacids under aqueous conditions.

Scheme 4. Aqueous Solid Phase Synthesis of b3-oligopeptides
3. A New Approach to b-Peptides Polymers
A particularly attractive application of this novel amide ligation without the need of activating the isoxazolidine building blocks is in the synthesis of polymeric peptides. Research in this area is currently in progress on identifying suitable isoxazolidines candidates for novel polymerization reactions. The synthesis of a mixture of isoxazolidine isomers 23 has been achieved, and preliminary results illustrate its ability to undergo polymerization following initiation with a ketoacid (Scheme 6). More stable and enantiomerically pure isoxazolidine monomers have also been prepared. The ability to grow polypeptides in water, in the absence of catalysts opens the door to new research opportunities in chemical biology and material science.[7]
![]()
Scheme 6. Poly-b-peptides by polymerizations of isoxazolidines
References
(1) Dawson, P. E.; Muir, T. W.; Clark-Lewis, I.; Kent. S. B. H. Science 1994, 266, 776-779.
(2) Dawson, P. E.; Kent. S. B. H. "Synthesis of Native Proteins By Chemical Ligations", Annu. Rev. Biochem. 2000, 69, 923-960.
(3) Bode, J. W.; Fox, R. M.; Baucom, K. W. " Chemoselective Amide Ligations by Decarboxylative Condenstationsof N-alkylhydroxylamines and a-Ketoacids", Angew. Chem. 2006, 118, 1270-1274.
(4) Carrillo, N.; Davalos, E. A.; Russak, J. A.; Bode, J. W. " Iterative, Aqueous Synthesis of b3 –Oligopeptides without Coupling Reagents" , J. Am. Chem. Soc. 2006, 128, 1452-1453.
(5) Vasella, A. "Stereoselectivity and Reactivity in 1,3-Dipolar Cycloaddition of Chiral N-(alkoxyalkyl) Nitrones", Helv. Chim. Acta,1977, 60, 1273–1295.
(6) Ramage, R.; Griffiths,G. J.; Shutt. F. E. "Dioxolanones as Synthetic Intermediates. Part 1. Sythesis of a- Keto Acids and a-Keto Aldehydes, and a- Ketols", J. Chem. Soc. Perkin. Trans. 1984, 1, 1531-1537.
(7) Cheng, J.; Deming T. J.; " Synthesis and Conformational Analysis of Optically Active Poly(b-peptides)", Macromolecules, 2001, 34, 5169-5174.