
ACS PRF | ACS
All e-Annual Reports

42944-G3
New Light-Harvesting Anionic Coordinating Borate Ligands for Lanthanide-Based Luminescence
This research proposal was initially focused on exploring the synthesis of new boron-containing ligands for studies on energy transfer in lanthanide complexes. As a result of PRF funding, a total of nine manuscripts have been accepted for publication on multiple subject areas and several more are in preparation. During the course of this project, an important new class of boron-containing fluorescent dyes has been discovered that permitted comprehensive studies centered on controlling energy transfer in multichromophoric arrays. While such derivatives may be useful for light-harvesting applications, we have focused our efforts in the opposite sense by exploring new ways to produce single molecule white-light emitters, a rare class of compounds that have enormous potential in energy-efficient lighting and display applications.
The newly discovered highly emissive fluorescent dyes are based on the Lewis acid complexes of 2-azolyl-anilines and are color tunable over the entire visible range More specifically, the first generation dyes are diphenylboryl-2-(pyrazolyl)aniline chelate complexes, or BORAZANS (Boron-Azo-Anilines) which are
Scheme 1. Preparation of 2-(pyrazolyl)aniline ligands and diphenylboron complexes. |
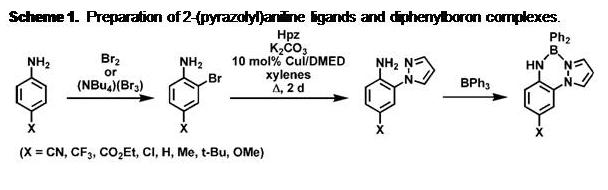 prepared according to Scheme 1 For convenience, a short-hand notation describes the substitution pattern on the 2-(pyrazolyl)anilines of the form pzxAny (where x and y represent substitution on the corresponding component; the un-substituted parent compound has no superscripts and an un-numbered superscript refers to substitution at the 4-position of the respective fragment). The ligands are not emissive, but the BORAZAN complexes are highly-emissive in non-Lewis basic solvents such as toluene (Figure 1). Excited state
prepared according to Scheme 1 For convenience, a short-hand notation describes the substitution pattern on the 2-(pyrazolyl)anilines of the form pzxAny (where x and y represent substitution on the corresponding component; the un-substituted parent compound has no superscripts and an un-numbered superscript refers to substitution at the 4-position of the respective fragment). The ligands are not emissive, but the BORAZAN complexes are highly-emissive in non-Lewis basic solvents such as toluene (Figure 1). Excited state Figure 1. Structure and emission of a blue, a cyan, and a yellow dye in toluene. |
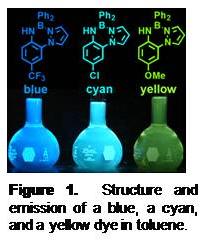 lifetimes of about 10 ns establish the fluorescent nature of emission. A main feature of these fluorophores is that the electronic properties and reactivity can be controllably tuned by varying the electron-donating or accepting properties of the substituent at the para- position of the aniline ring. Thus, electron-withdrawing substituents (such as CN or CF3 groups) lead to BORAZAN derivatives that give higher quantum yields (FF ca 0.8 in toluene) and higher-energy (blue) emission compared to more electron donating groups such as OMe which gives red-shifted (yellow) emission with a lower quantum yield (FF ca 0.07 in toluene). Electron-withdrawing groups render the boron more electrophilic, resulting in stronger and shorter B-N(pyrazolyl) bonds compared to derivatives with electron-donating para-aniline substituents. As such, the electron-deficient anilines are more resistant than electron-rich aniline derivatives toward solvolysis by protic solvents. Also, the fluorescent quantum yields drop more dramatically in CH3CN for electron donating substituents (X = OMe; FF = 0.002 in CH3CN) versus (X = CN; FF = 0.74 in CH3CN) which can be attributed to the Lewis acid/base reactions of the dyes with solvent.
lifetimes of about 10 ns establish the fluorescent nature of emission. A main feature of these fluorophores is that the electronic properties and reactivity can be controllably tuned by varying the electron-donating or accepting properties of the substituent at the para- position of the aniline ring. Thus, electron-withdrawing substituents (such as CN or CF3 groups) lead to BORAZAN derivatives that give higher quantum yields (FF ca 0.8 in toluene) and higher-energy (blue) emission compared to more electron donating groups such as OMe which gives red-shifted (yellow) emission with a lower quantum yield (FF ca 0.07 in toluene). Electron-withdrawing groups render the boron more electrophilic, resulting in stronger and shorter B-N(pyrazolyl) bonds compared to derivatives with electron-donating para-aniline substituents. As such, the electron-deficient anilines are more resistant than electron-rich aniline derivatives toward solvolysis by protic solvents. Also, the fluorescent quantum yields drop more dramatically in CH3CN for electron donating substituents (X = OMe; FF = 0.002 in CH3CN) versus (X = CN; FF = 0.74 in CH3CN) which can be attributed to the Lewis acid/base reactions of the dyes with solvent. In order to improve on the dye design, a second generation BORAZAN dye was developed in which two pyrazolyl rings were bound to the aniline rings, as in Scheme 2. The
Scheme 2. Preparation of di-2,6-(pyrazolyl)aniline ligands and diphenylboron complexes.
|
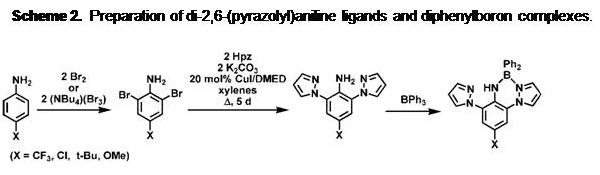 advantage of di-pyrazolyl substitution is that the dye framework is (kinetically) more stable than the mono-pyrazolyl counterparts. Indeed, the dipyrazolyl BORAZANs BPh2(pz2AnX) are stable toward solvolysis and can even be purified by column chromatography on silica gel, a process that destroys the mono-pyrazolyl BORAZANs, BPh2(pzAnX). Replacing the second ortho-hydrogen of a mono-pyrazolyl BORAZAN with a more electron donating pyrazolyl in the BPh2(pz2AnX) results in a red-shifted absorption/emission compared to BPh2(pzAnX). Despite the red-shifted emission, there is an increase in fluorescence quantum yield in toluene of a given BPh2(pz2AnX) compared to the corresponding BPh2(pzAnX) (for example FF = 0.46 for BPh2(pz2AntBu) but FF = 0.33 for BPh2(pzAntBu)). More importantly, the fluorescence quenching effect of CH3CN is lessened for the di-pyrazolyl than the mono-pyrazolyl derivatives (for example, FF = 0.16 for BPh2(pz2AntBu) but FF = 0.03 for BPh2(pzAntBu) in this solvent).
advantage of di-pyrazolyl substitution is that the dye framework is (kinetically) more stable than the mono-pyrazolyl counterparts. Indeed, the dipyrazolyl BORAZANs BPh2(pz2AnX) are stable toward solvolysis and can even be purified by column chromatography on silica gel, a process that destroys the mono-pyrazolyl BORAZANs, BPh2(pzAnX). Replacing the second ortho-hydrogen of a mono-pyrazolyl BORAZAN with a more electron donating pyrazolyl in the BPh2(pz2AnX) results in a red-shifted absorption/emission compared to BPh2(pzAnX). Despite the red-shifted emission, there is an increase in fluorescence quantum yield in toluene of a given BPh2(pz2AnX) compared to the corresponding BPh2(pzAnX) (for example FF = 0.46 for BPh2(pz2AntBu) but FF = 0.33 for BPh2(pzAntBu)). More importantly, the fluorescence quenching effect of CH3CN is lessened for the di-pyrazolyl than the mono-pyrazolyl derivatives (for example, FF = 0.16 for BPh2(pz2AntBu) but FF = 0.03 for BPh2(pzAntBu) in this solvent).We have examined different methods of producing white light using our dyes, including making physical mixtures (both in solution and in the solid state), as shown for a toluene solution in Figure 2. The successful implementation of this strategy (and resulting spectrum) provides the benchmark for broad-band emission (the lack of energy transfer) when evaluating the spectrum of covalently-linked dyes. All of the ligands undergo useful derivatization reactions that can be exploited in order to covalently link the chromophores into dyads or higher-order assemblies. For instance,
Figure 2. White light emission obtained by UV irradiation of a mixture of blue and yellow dyes in toluene, with calculated and observed absorption spectra (right).
|
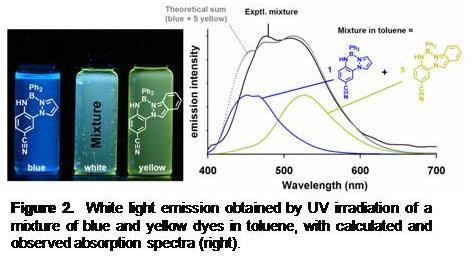
Figure 3. Preparation and spectrum of a directly-linked blue/cyan BORAZAN, BPh2(pzAnCF3)-BPh2(pzAnMe). |
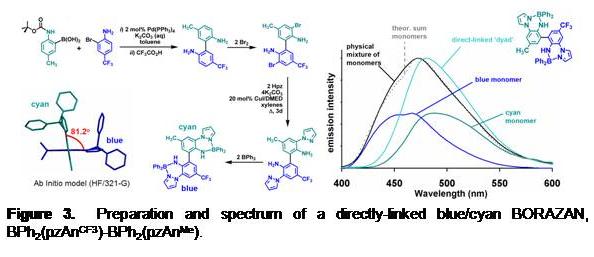 H(pzAnX) undergoes selective bromination at the ortho-aniline position (with 1 equiv of brominating reagent) that is then susceptible to Pd-catalyzed C-C and C-N coupling reactions. In this way several ‘dyads' (exemplified by the one in Figure 3) have been prepared for further evaluation in energy transfer.
H(pzAnX) undergoes selective bromination at the ortho-aniline position (with 1 equiv of brominating reagent) that is then susceptible to Pd-catalyzed C-C and C-N coupling reactions. In this way several ‘dyads' (exemplified by the one in Figure 3) have been prepared for further evaluation in energy transfer.