ACS PRF | ACS
All e-Annual Reports

42964-AC1
Asymmetric Hydrocyanation of Olefins
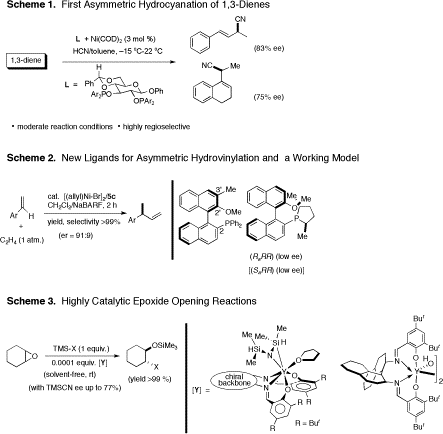
Asymmetric Hydrocyanation of 1,3-Dienes. 1,2-bis-Diarylphosphinites are excellent ligands for the Ni(0)-catalyzed hydrocyanation of certain types of 1,3-dienes. 1-Phenylbuta-1,3-diene, 1-vinyl-3,4-dihydronaphthalene and 1-vinylindene undergo highly regioselective hydrocyanation under ambient conditions to give exclusively the 1,2-adducts in good to excellent yields. Using bis-1,2-diarylphosphinites derived from D-glucose the highest enantioselectivities to-date for asymmetric hydrocyanation of 1,3-dienes (70-83% ee's) have been obtained. The most salient features of this work are: 1. A new, highly selective protocol for Ni-catalyzed hydrocyanation of dienes at low temperatures has been developed (yields 71-95%, regioselectivity approaching 99% for several different dienes. Reactivity differences between these dienes are also illustrated. 2. An asymmetric variation of this reaction gives good yields and ee's in the range of 70-83% for selected substrates. Since the cyano group can be readily converted into other functional groups, this is a potentially useful reaction for the synthesis of valuable intermediates from readily available starting materials. 3. Surprising formation of an all-carbon quaternary center, with possible mechanistic implications of a remote participation by a pendant alkene ligand. (Scheme 1) Ligand Effects in Asymmetric Catalysis. Study of ligand effects in hydrovinylation, a reaction related to asymmetric cyanation, lead us to propose a working model for the induction of asymmetry in that powerful reaction. Among the handful of monophosphine ligands that effect asymmetric hydrovinylation of vinylarenes, 2-diphenylphosphino-2'-methoxy-1,1'-binaphthyl (MOP) is among the most accessible. Addition of a methyl group at the 3'-position of this ligand significantly improves the enantioselectivity of hydrovinylation of prototypical alkenes. Introduction of a chiral phospholane at C2 position of this scaffolding has no effect on the enantioselectivity. . These results are consistent with a model proposed for the asymmetric induction for this exacting reaction. (Scheme 2) Highly Catalytic Epoxide Opening Reactions. Halide or alkoxide free-yttrium salen complexes are excellent catalysts for the ring opening of epoxides mediated by TMSCN and TMSN3. Substrate to catalyst ratios up to 10,000 has been realized in these potentially useful reactions, which can be run under solvent-free conditions. Even though the enantioselectivities for the TMSCN-mediated reaction remains modest (best 77% ee), these studies with a highly tunable ligand system may provide further impetus for work in this important area of catalysis. Even though attempts to isolate a Y-cyanide complex, which was detected by in situ IR spectroscopy, failed, monomeric and dimeric yttrium complexes with possible relevance to this highly catalytic reaction were isolated and characterized by spectroscopy and X-ray crystallography. A kinetic study using in situ IR spectroscopy did not provide conclusive data to assign an order with respect to Y in this reaction. (Scheme 3) We are continuing to look for applications of the asymmetric hydrocyanation and hydrovinylation in a novel solution to the classical problem of controlling exocyclic stereochemistry. We will shortly report an application for the synthesis of much sought after steroid derivatives with 20(S)-configuration.