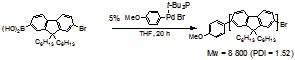ACS PRF | ACS
All e-Annual Reports

44428-AC1
Controlled Pd(0)-Catalyzed Cross-Coupling Polymerizations
Aimed at developing controlled Pd-catalyzed cross-coupling polymerization processes, we have focused our research on the following two specific aims in the past year: (a) to understand whether the “Preferential Oxidative Addition” mechanism can be applied for the cross coupling of 1,2-dihalobenzenes with Gringard reagents, and (b) to test the feasibility of controlled palladium-catalyzed Suzuki cross-coupling polymerization.
In our preliminary study, we have established that dibromobenzenes and diiodobenzenes can coupling with arylboronic acids via “Preferential Oxidative Addition” mechanism with Pd(0)/t-Bu3P as catalyst. To gain information about whether the cross-coupling of 1,2-dihalobenzenes with other organometallic reagents can also occur via “Preferential Oxidative Addition” mechanism, we tested the reactions of 1-chloro-2-halobenzenes and 2-haloaryl tosylates with Grignard reagents including 2,6-dimethylphenylmagneisum bromides. We found “Preferential Oxidative Addition” phenomena exist when palladium catalysts with phosphine and N-heterocyclic carbene ligands were used. We observed that good to excellent yields of substituted fluorenes were obtained when palladium catalysts without phosphine and N-heterocyclic carbene ligands, e. g., Pd(OAc)2, were used (Scheme 1). The formation of substituted fluorenes suggested that benzynes were likely involved as the reaction intermediates. Our study provides the synthetic community an efficient method for the preparation of substituted fluorenes, which are potentially useful building blocks in polymer/materials fields, from readily available starting materials.
Scheme 1.
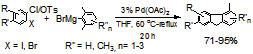
We extended the benzyne intermediate hypothesis by envisioning carbon-carbon triple bonds should function similarly as those in the benzyne intermediates. We tested the reactions of internal alkynes with 2,6-dimethylphenylmagnesium bromides. We found with Pd(OAc)2 as catalyst, substituted indenes were obtained in good to excellent yields, and with Pd(OAc)2/PPh3 as catalyst, (Z)-tetra-substituted ethenes were obtained in good yields. Our study validated the benzyne intermediate hypothesis for the Pd(OAc)2-catalyzed reaction of 1,2-dihalobenzenes with hindered Grignard reagents and provides efficient methods for the preparation of two types of useful organic compounds.
Scheme 2.

We have also tested Pd(0)/t-Bu3P-catalyzed cross-coupling polymerization of a fluorene-containing monomer. We chose this polymerization because polyfluorenes have been demonstrated to be promising light-emitting materials and 2,7-dibormofluorenes also underwent cross-coupling with arylboronic acids via “Preferential Oxidative Addition” mechanism. With 4-CH3OC6H4Pd(t-Bu3P)Br as initiator, we found the polymerization went well and the polymer obtained has the molecular weight of Mw = 8 800 (PDI=1.52) (polystyrene as standard, THF) (Scheme 3). Our study suggested that controlled Pd(0)-catalyzed Suzuki cross-coupling polymerization could be possible. We are now optimizing the polymerization condition to better control the polymerization process.
Scheme 3.