
ACS PRF | ACS
All e-Annual Reports

44160-AC4
Investigation of Dye-Protein Interactions and Optimization of Fluorescence-Based Assays for Target Binding of Calmodulin
Chemists and biologists frequently turn to fluorescence techniques to probe protein interactions and dynamics. These techniques often rely on a fluorescent dye covalently attached to the protein. The nature of interactions between the dye and the protein are important for the reliability of these assays. The goals of this project are (1) to characterize the influence of electrostatic interactions on the interactions of dyes attached to the protein calmodulin (CaM), and (2) to use this knowledge to develop sensitive assays for target binding by CaM. Dye-protein interactions in CaM. The fluorescence lifetime and fluorescence anisotropy decays were measured for CaM labeled with three fluorescence dyes bearing different charges by time-correlated single photon counting. The following dyes were chosen in this experiment.
Dye | Charge | Wavelength abs/emission (nm) | rotational correlation time (ns) | Fraction |
Alexa Fluor488 | -1 | 453/460 | 0.19 | 0.51 |
|
|
| 3.59 | 0.49 |
Oregon Green 488 | 0 | 475/460 | 0.20 | 0.50 |
|
|
| 4.18 | 0.50 |
Atto465 | 1 | 475/460 | 0.73 | 0.29 |
|
|
| 7.95 | 0.71 |
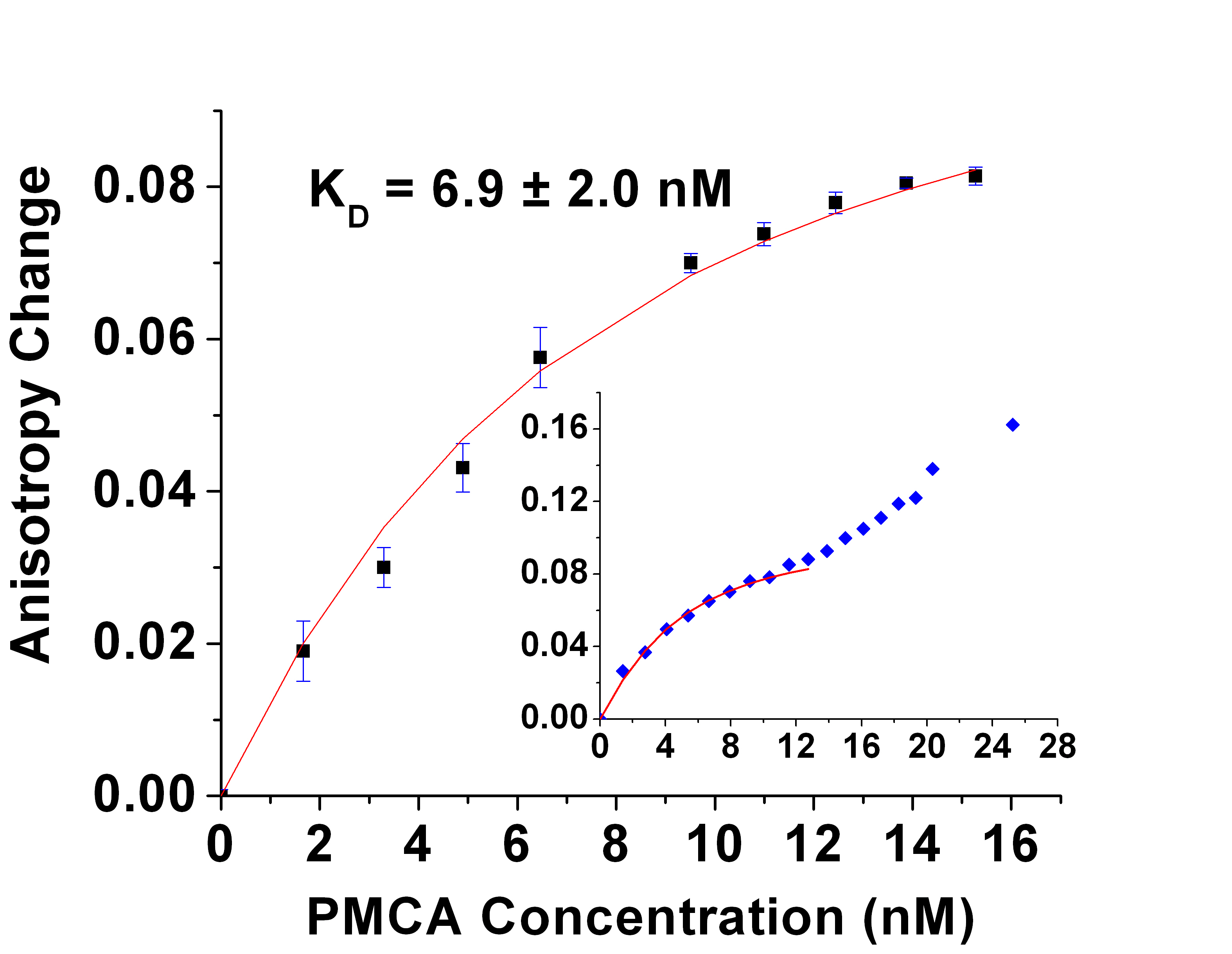
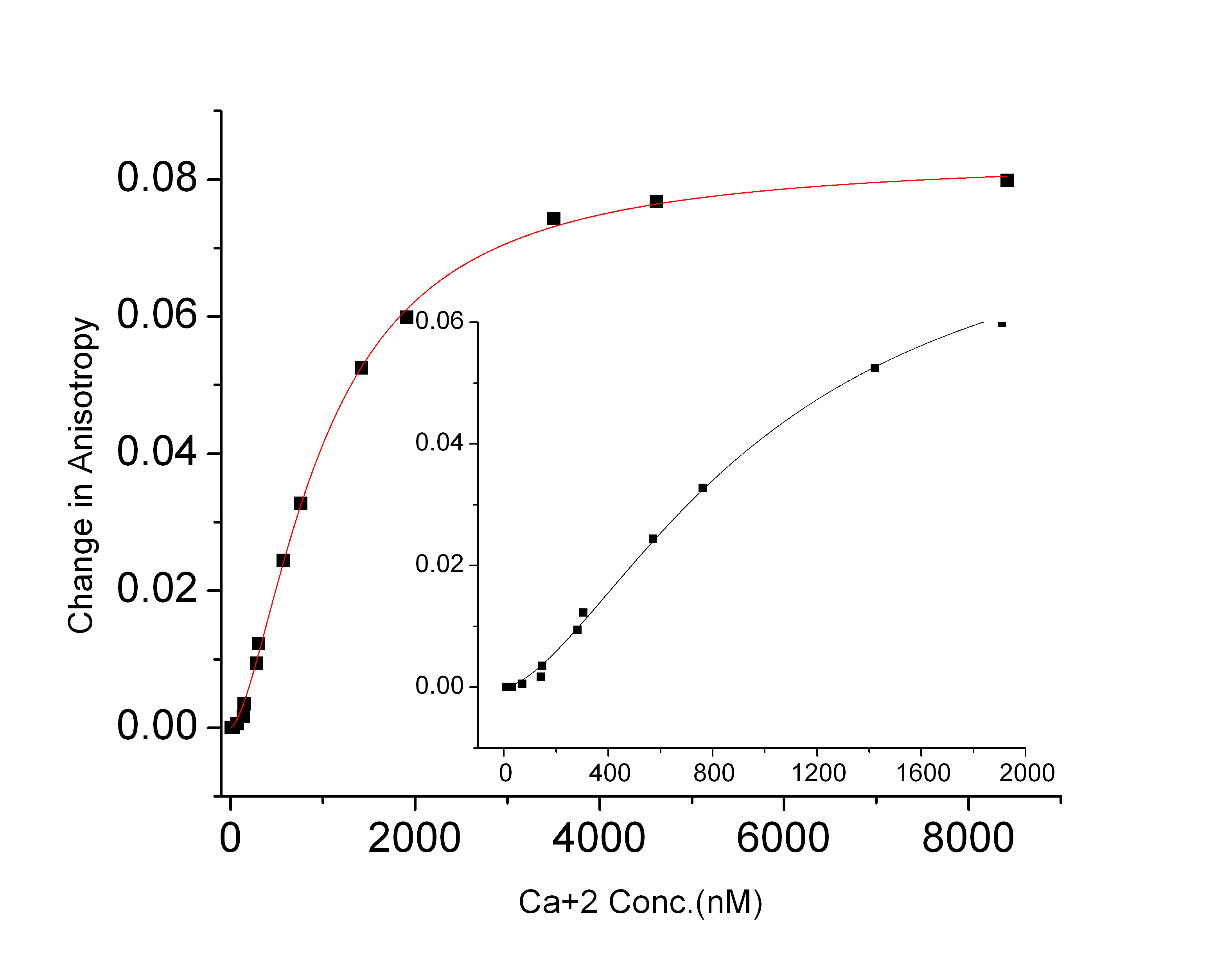
constant Kd for the CaM- PMCA complex. The result shown in the figure yields the binding affinity of CaM-PMCA (Kd = 6.9 nM). As the PMCA concentration was increased above 12 nM, a further increase in the anisotropy indicated aggregation of the protein. The second figure shows the results of FP measurements as a function of Ca2+ concentration have similarly yielded the Ca2+ affinity of CaM-PMCA complexes. The dependence at Ca2+ concentrations below 200 nM shows cooperativity in Ca2+ binding (Hill coefficient 1.6). A manuscript has been submitted describing these results.