ACS PRF | ACS
All e-Annual Reports

45651-AC4
Novel Topologies for Unpaired-Electron Interactions
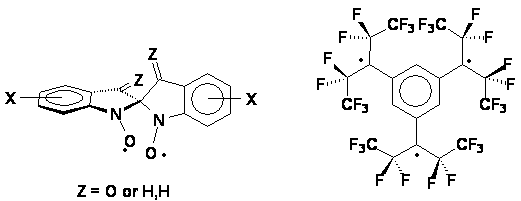
| We are preparing new di- and tri-radicals that allow for new topologies of intramolecular and intermolecular spin interactions. In one category of radicals, the new species are designed by combining two identical radical subunits in such a way as to allow for their singly-occupied molecular orbitals to spiroconjugate, giving two new molecular orbitals spanning the entire molecule. The new topology yields species with electrons delocalized in two perpendicular planes. The new species under investigation include spiroconjugated nitroxyl radicals. The second category involves tertiary radicals with large perfluoroalkyl groups. The perfluoroalkyl groups furnish steric barriers to the undesired bond-forming reactions, and provide the opportunity to explore intramolecular spin-spin interactions between orbitals of parallel and perpendicular topologies. The radicals are constructed in such a way as to have their radical centers buried inside and have perfluorinated alkyl groups exposed to the outside - not unlike molecular "teflon balls". The new species under exploration include the "perfluoro" triradical.
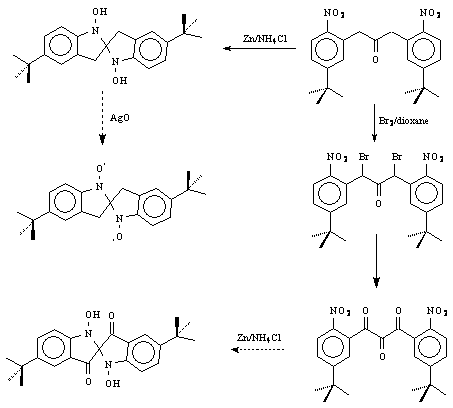
The synthetic approach to the dinitroxides is illustrated by our work to date on p-t-butyl derivative (used to increase the persistence of the diradical). The synthesis started with m-t-butyltoluene. The intermediate ketone (see below) was formed by the reaction of the benzylic anion with ethyl formate, followed immediately by PCC oxidation. Nitration gave the desired dinitro(mono)ketone, which was then brominated with Br2 in dioxane to give the dibromonitroketone. The silver-assisted hydrolysis/oxidation to the corresponding triketone was carried out in DMSO. We plan to reduce the dinitrotriketone to the corresponding spirodihydroxylamine, which will be the direct precursor to the nitroxyl diradical (after oxidation with AgO). We have been able to ascertain that the scheme is workable, by reduction of the dinitro(mono)ketone to the corresponding (Z = H,H) hydroxylamine with Zn in the presence of a week acid. That hydroxylamine will also be oxidized to the diradical.
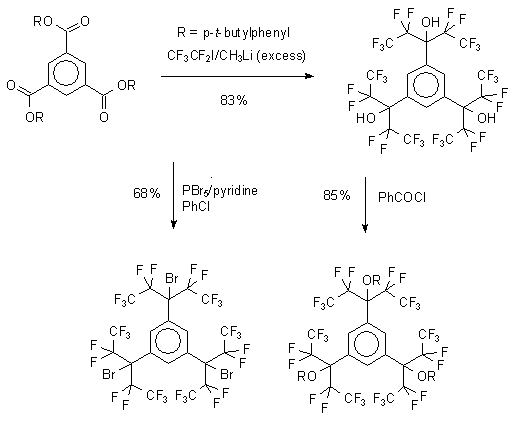
The synthesis of “perfluoro” radical precursors has been accomplished in a couple of steps from commercially available starting materials. The versatility of the synthetic route is illustrated on the example of precursors to the tri-radical, one of the most challenging targets within this series. The trialcohol was prepared in high yield (97% per addition step) via a six-fold addition of the perfluoroethyllithium. The addition can be halted at the ketone stage, thus providing substrates susceptible to further modification. The alcohol was converted to the corresponding tribromide which serves as the direct precursor to the tri-radical). The tri-alcohols has been converted successfully to selected other derivatives, such as ethyl oxalates, N,N-dimethylthiocarbamates, chloroformates and benzoates. The first three derivatives may yield the corresponding radicals under the standard conditions, and the benzoate is the precursor to the corresponding hydrocarbon in the unique, photoinduced electron-transfer reaction.
|