ACS PRF | ACS
All e-Annual Reports

44286-AC1
Bio-Inspired Cobalt Catalysts for Carbene and Nitrene Transfer Reactions
1. Design and Synthesis of New Chiral Porphyrins
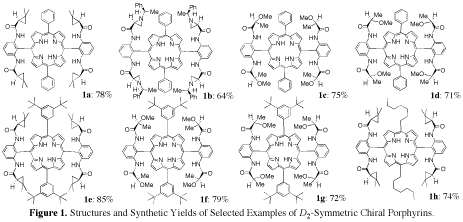
Using readily accessible bromoporphyrins, we have synthesized a series of chiral porphyrins via palladium-mediated quadruple amidation reactions with a set of commercially available chiral amide building blocks. These D2- or pseudo-D2-symmetric chiral porphyrins possess varied electronic, steric, and chiral environments. Some of the examples are shown in Figure 1.
2. Asymmetric Cyclopropanation of Alkenes Among the family of D2-symmetric chiral porphyrins we have synthesized (Figure 1), [Co(1e)] has emerged as one of the best chiral catalysts for asymmetric cyclopropanation of alkenes because of its low catalyst loading, mild reaction conditions, practical operation protocol, high product yield, exceptional trans-diastereoselectivity, and excellent enantioselectivity. We have been in the process of thoroughly exploring the substrate scope and limitation of the [Co(1e)]-based catalytic system by examining a wide range of alkenes in combination with different diazo reagents. Some of the selected results are summarized in Table 1. It is noteworthy that even the extremely electron-deficient pentafluorostyrene could be cyclopropanated, albeit in relatively lower yields (entries 33–35). In most of above cases (Table 1), corresponding trans-cyclopropane esters were produced with excellent diastereoselectivity and enantioselectivity. Although the catalytic reactions were carried out with styrenes as the limiting reagents and without slow addition of EDA or t-BDA, it is notable that little to no dimerization side products from the diazo reagents were observed. In addition to the aforementioned case of pentafluorostyrene, the electron-deficient a,b-unsaturated carbonyl compounds and nitriles have been a class of challenging substrates for cyclopropanation. Few catalytic systems can effectively cyclopropanate this class of alkenes. Our recent studies indicated the [Co(1e)]-based system can catalyze diastereoselective and enantioselective cyclopropanation of electron-deficient a,b-unsaturated carbonyl compounds and nitriles with diazoacetates, provide the desired cyclopropanes in high yields and selectivities. We are in the process of completing this exciting work. 3. Selective Aziridination of Alkenes Previously, we reported that iron and cobalt porphyrins could effectively catalyze aziridination reactions of a wide variety of alkenes with bromamine-T as an alternative nitrene source. Recently, we revealed that cobalt(II) porphyrin complex such as Co(TPP) can catalyze aziridination of alkenes using diphenylphosphoryl azide (DPPA) as a convenient new nitrene source, leading to the formation of N-phosphorylated aziridines with dinitrogen as the by-product (Equation 1). Employing the D2-symmetric chiral porphyrins 1 (Figures 1), we have carried out systematic studies aiming to develop asymmetric versions of these catalytic aziridination processes. While no asymmetric induction has been observed in the bromamine-T system, significant enantioselectivities have been obtained for Co-catalyzed aziridination with DPPA. Furthermore, it was found that [Co(1)] complexes are much more active catalysts than Co(TPP), allowing aziridination of alkenes with DPPA to proceed under milder conditions with higher yields. During the study, we also observed some large solvent effect. Together with the use of other derivatives of phosphorus-based azides, we are in the process of designing and synthesizing new chiral porphyrins to further improve the asymmetric aziridination system.