ACS PRF | ACS
All e-Annual Reports

45092-GB1
Synthesis and Transition Metal-Catalyzed [3+2] Cycloadditions of Methyleneaziridines
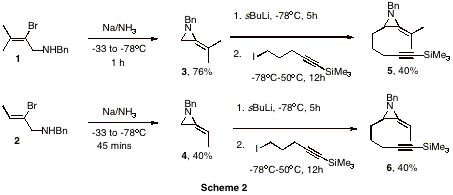
The main goal of this research was to prepare tethered methyleneaziridines and investigate their transition-metal catalyzed cycloadditions. Work in the first year has focused on the synthesis of the required methyleneaziridines and their tethering reactions. Initial work has also begun on the cycloaddition process that was proposed. The precursors to the methyleneaziridines currently being tethered are 1 and 2 (Scheme 1) and these have been prepared on a multi-gram scale.
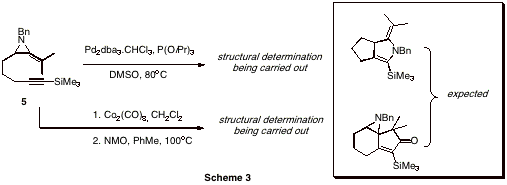
The cyclization of 1 and 2 to the methyleneaziridines is not a trivial process and requires generation of sodium amide in liquid ammonia.[1] Significant time was spent training three undergraduate researchers to be proficient in this reaction and we are now able to routinely produce grams of the methyleneaziridines at a time. The resulting methyleneaziridines 3 and 4 were then tethered with an alkyne chain via the lithium anion of the parent methyleneaziridines to yield novel cyclization substrates 5 and 6 (yields un-optimized). An extensive screen of palladium/ligand combinations was carried out using parallel synthesis techniques. While the cyclization substrates 5 and 6 have found to be unreactive to Pd(PPh3)4, electron-poor phosphites in conjunction with Pd2(dba)3.CHCl3 yielded a yet unidentified product. Work is currently being carried out to scale up this apparent cyclization in order to allow a full structure elucidation. In addition to palladium-catalyzed reactions we also began investigating cobalt-mediated Pauson-Khand reactions of our tethered substrates. Again, the spectral data suggest a cyclization has occurred but not to the expected product. We anticipate to have elucidated the structures for both these reaction-products in the very near future. Other work has looked at the reaction of the aziridinyl-anion with aldehydes (Scheme 4). In this case, the product from the reaction contains two stereogenic centers, so the formation of diastereoisomers is possible. In the case of the reaction of 3 with benzaldehyde, we obtained 7 as a 2:1 ratio of diastereoisomers. The product is crystalline and we are currently growing crystals of x-ray quality to unambiguously determine the stereochemistry. We rationalized that as methyleneaziridines have been shown to undergo ring-opening under Lewis-acidic conditions to aziridinium ions, that 7 should form 9 under such conditions.[2] Azriridinium ion 9 may then undergo nucleophilic attack by the pendant oxygen, yielding dihydrofurans. Indeed, we have found this to be the case and have very recently obtained 8 from the BF3.OEt2 mediated rearrangement of 7. This process is as yet unoptimized, but shows proof of principle. Work will continue to synthesize a range of analogues of 7 and investigate their rearrangement to the interesting dihydofuran skeleton.