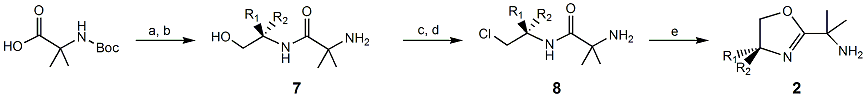ACS PRF | ACS
All e-Annual Reports

44908-GB1
Building Enantioselective Ligands: New Frontiers with Oxazolines
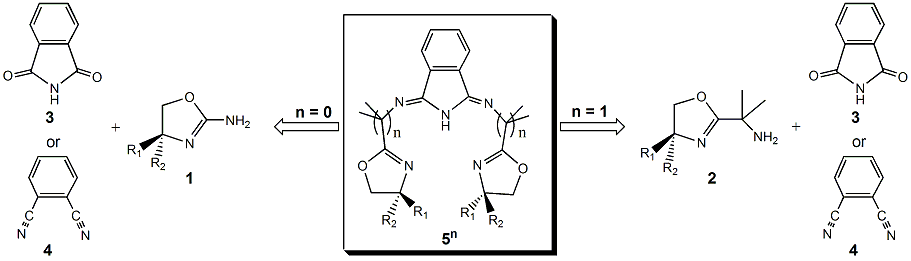
Our research group has made good progress on the synthesis of our proposed bis(oxazoline)-isoindoline ligand system 5n (n= 0,1). A summary of the progress is outlined below. We have optimized the protocols for the synthesis of the synthons for 50; 1-Me2 can be prepared in 70% and chiral 1-(H)(Ph) can be prepared in 88% yield. Each of these synthons have undergone a battery of coupling reactions with both 3 and 4 in order to try and obtain the proposed 50-Me2 and chiral 50-(H)(Ph) ligands. Attempts to form the ligands using literature methods, which condense 4 with amines at high temperatures in alcohol solvent, were unsuccessful as synthons 1 were found to slowly decompose at elevated temperatures. Less severe conditions were used based on the protocol reported by Gómez et al. (JCS Dalton, 2001) for the formation of 1,2-bis(oxazole)benzene where ZnCl2 was a catalyst. After chromatography to purify the product, the free ligand was not isolated but rather the air-stable bright yellow (50-(H)(Ph))ZnIICl (6) was obtained. 1H NMR analysis indicated that the desired ligand 50-(H)(Ph) had formed based on the number of signals, couplings, and integration. Atomic absorption spectrometry confirmed the presence of zinc and electrospray mass spectrometry yielded a parent ion peak that corresponds to the [Zn(50)]+ fragment. Overall we are excited about the successful isolation of a 51 ligand as a zinc complex. Efforts are being made to optimize the synthetic yield of the product and to isolate the free ligand.
In the case of the synthons for 51, we have successfully prepared and optimized the protocols to make 2-(H)(Ph) and 2-(H)(iPr). We changed our original design from a glycine-based primary amine end to a dimethyl glycine-based tertiary amine. The change was made once it was discovered that the addition of the two methyl groups greatly improved the solubility of 7-(N-Boc protected) in nonpolar solvents and resulted in overall better yields.
The synthesis of 7 was optimized using an HBTU-assisted coupling reaction as the reaction does not require the use of flash chromatography to purify the product. After the removal of the Boc protecting group with TFA to yield 7, two methods were successful in its cyclization to 2; (i) activation using SOCl2 followed by base-induced cyclization and (ii) concomitant activation and cyclization of the hydroxylamine using DAST. Owing to the higher yields obtained with SOCl2 and as DAST is much more expensive, we have chosen to employ the SOCl2 method from here on. We have successfully prepared 2-(H)(iPr) and 2-(H)(Ph). Attempts to couple these synthons to 3 or 4 are currently underway.
All of the research was performed by two undergraduate students at