ACS PRF | ACS
All e-Annual Reports

40443-B1
The Palladium-Mediated Cyclization of Beta-Allenyl Oximes
The palladium-mediated nucleometalation / methoxycarbonylation of a β,γ-unsaturated oxime has been shown to provide a racemic mixture of a 3,5-disubstituted Δ2-isoxazoline. In addition, we have also shown that the α,β-unsaturated ketone can undergo a tandem oximation-ring closure to provide 3,5-disubstituted Δ2-isoxazolines. These novel routes to the preparation of an isoxazoline ring system are stable to a wide variety of substituents. In addition, they provide the desired products with complete control of regiochemistry and a potential for complete control of the stereochemistry of the isoxazoline at C5.
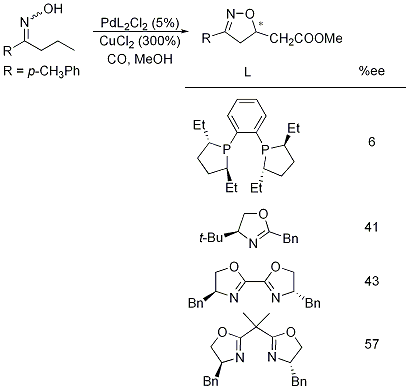
Specifically, in the last year, we have focused our efforts on the development of a method to prepare the Δ2-isoxazoline ring system with enantiomeric control. The use of chiral ligands with the palladium-mediated cyclization has provided some success in this area. Specifically, we have explored the use of commercially available chiral ligands to direct the addition of the oxime's hydroxyl group to a particular face of the alkene. The results of our efforts thus far indicate that increasing steric bulk on a bis-oxazoline ligand does provide the necessary steric hindrance to limit attack on one face of the alkene (see table below). Our current efforts have been directed toward the preparation of bulkier ligands in order to fully realize the potential enantiomeric control in the palladium-mediated cyclization.
In addition, we have developed a route to the Δ2-isoxazoline ring system in two steps from an aldehyde and a ketone. In the first step, the aldol condensation of the two starting materials provides the substituted chalcone in excellent yield. Simple recrystallization is done to isolate and purify the α,β-unsaturated ketone. In the second step, warming the ketone in a solution of hydroxylamine hydrochloride in the presence of a strong base results in the tandem oximation – cyclization to give rise to a 3,5-disubstituted Δ2-isoxazoline. This compound is isolated and purified by flash chromatography. We are currently exploring the use of chiral bases to effect enantiomeric ring closure in the tandem reaction.