ACS PRF | ACS
All e-Annual Reports

39421-B4
Perturbations Upon Aromaticity and Antiaromaticity Due to Isotopic Substitution
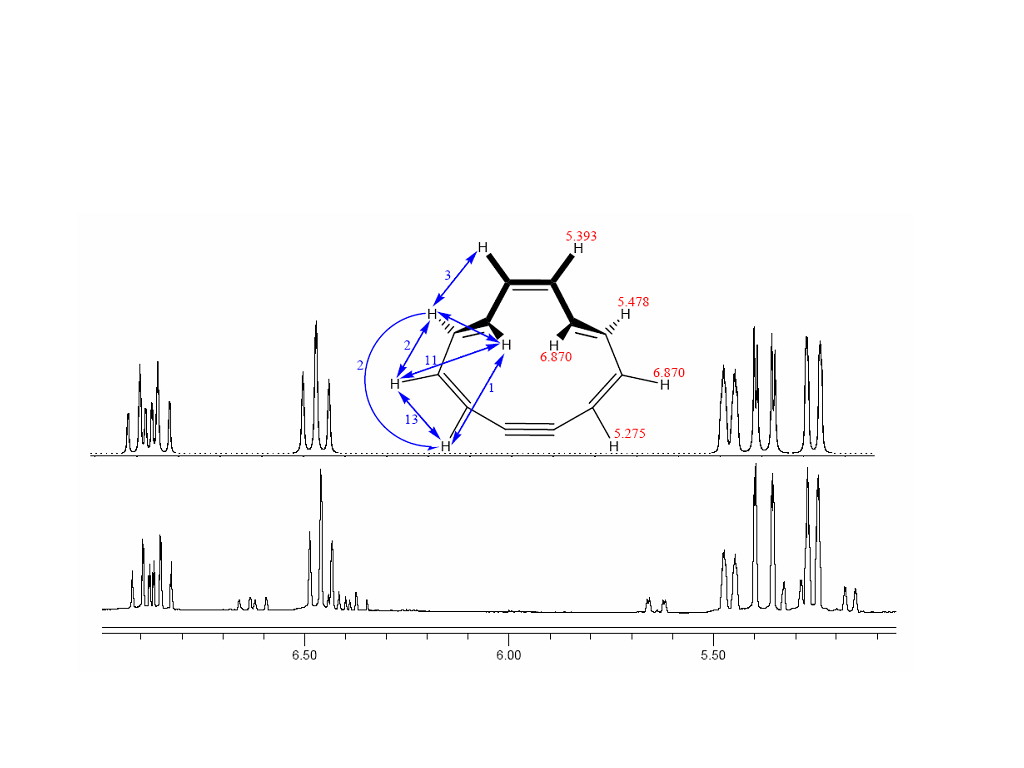
When a THF-d8 solution of hexadiyne (1), in an evacuated apparatus, is treated with a molar excess of potassium tert-butoxide, a deep purple solution is formed. The volatiles can be distilled directly into an attached NMR tube by simply cooling the NMR tube. The resulting clear colorless solution reveals an NMR pattern due to a 1:1 mixture of the two known isomers of [12]annulyne (2 and 3). When this solution comes into contact with a freshly distilled potassium metal mirror, the solution becomes paramagnetic and reveals a strong well resolved EPR signal of the heptalene anion radical. From this, it seems that the equilibrium: 2.- 3.- is established, and one of the anion radicals quickly rearranges. On the other hand, it is possible that both quickly rearrange to heptalene.-. To resolve this dilemma, we carried out a flash pyrolysis of the 1:1 mixture of 2 and 3 by simply distilling the mixture through a short tube (see experimental) that was warmed to 150 oC. The NMR spectrum shows that heating increased the [2]/[3] ratio, and the new ratio of materials remains constant for over 24 hours. Hence, equilibrium between 2 and 3 must take, at least, days to be established, which is not unexpected due to the rigidity that is enforced by the triple bond. Since the reduction of a 1:1 mixture of 2 and 3 yields an immediate unchanging concentration of heptalene anion radical, most likely both 2.- or 3.- instantly (within seconds) rearrange to the anion radical of heptalene. All three are members of the C12H10.- manifold, so the mechanism can only involve hydrogen migrations and rehybridizations. Due to the considerable strain on the triple bond, [6]Annulyne is often written as a diradical, and there is more strain on the triple bond in 3 than in 2. Consequently, 3 can be thought to have some diradical character, and its reduction leads to heptalene.-. It is curious and unknown why the hydrogen migration and ring closure is such as to yield heptalene.- as opposed to the anticipated benzocyclooctatetraene.-. However there does appear to be some natural pressure to form heptalene.-; for example: the anion radical forms spontaneously from the di-trans-[12]annulene anion radical. The ratio of 2 to 3 produced by the action of potassium tert-butoxide in THF is very close to 1. However, this ratio, does depend upon the base used, and it is x/1 when the potassium tert-butoxide is replaced by sodium acetylide. We felt that the nature of the ion association in the base affects the [12]annulynes produced. For this reason, the base condensation of hexadiyne was carried out with potassium tert-butoxide in perdeuteriated benzene (C6D6), a non-polar solvent. This condensation produces an entirely different isomer of [12]annulyne, which also yields five resonances integrating 2:2:2:2:2, Figure 1. There are also many low intensity resonances present, of which some may be due to another minor isomer of [12]annulyne. Figure 1. (Lower) The 1H-NMR spectrum obtained from the base {KOC(CH3)3} initiated condensation of hexadiyne in C6D6. (Upper) A computer simulation (of the major species) generated using the J splittings (blue in Hz) and chemical shifts shown (red in ppm). The minor isomer has yet to be identified. One electron of this new isomer of [12]annulene results in the formation of the anion radical of biphenyl.