ACS PRF | ACS
All e-Annual Reports

44568-B5
Electrodeposition of Metal Alloy and Mixed Oxide Films Using a Single-Precursor Tetranuclear Heteropolymetallic Complexes
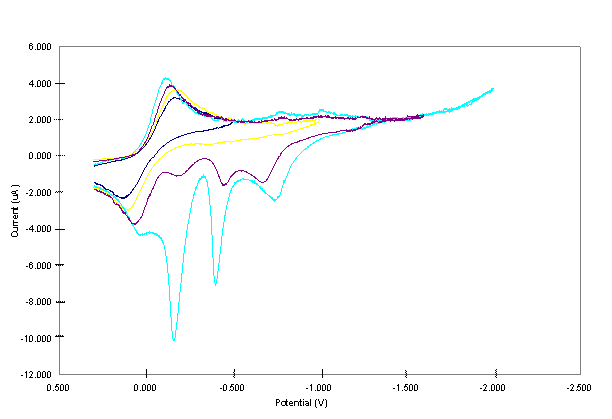
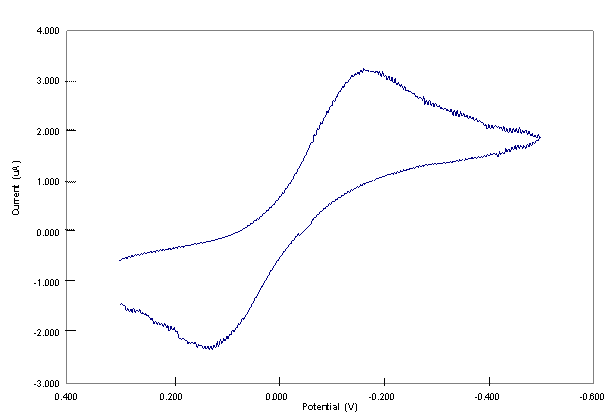
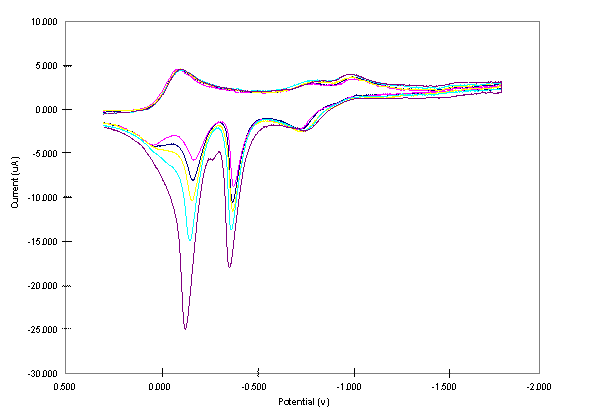
At the initial stage of the project our primary objective was to organize the laboratory, and introduce the students with the various experimental skills necessary for the synthesis and purification of the complexes. To achieve this goal, we performed previous transmetallation works on Cu2Ni2, Cu3Co, and Cu2Co2 complexes.1-3 The purity of the complexes were checked using their electronic spectra. Cu2Ni2 was synthesized in methylene chloride by transmetallation according to (µ4-O)(denc)4Cu4Cl6 + 2Ni(NS) 2 → (μ4-O)(denc)4Cu2(Ni(H2O))2Cl6 + 2Cu (NS) 2 (1) where NS = S-methyl isopropylidenehydrazinecarbodithioate.1-3 An additional water molecule is coordinated to the Ni center of Cu2Ni2 during its purification. The Cu(NS)2 was separated from Cu2Ni2 by gel permeation chromatography. (µ4-O)(denc)4Cu4Cl6 [Cu4] was prepared by treating a solution of CuCl2.2H2O with a solution of denc, both in methanol, and refluxing the reaction mixture and adding sodium methoxide solution in methanol.4 Ni(NS)2 was obtained by treating a solution of HNS with a solution of Ni(acetate)2.4H2O, both in ethanol, and refluxing the reaction mixture.5 Cu3Co and Cu2Co2 were synthesized and purified following the procedure of Cu2Ni2 using Co(NS)2 as a transmetallator. Co(NS)2 was prepared using the same procedure to prepare Ni(NS)2 except that all the solvents used were flushed with nitrogen prior to use.5 Further studies on Cu2Co2 were not conducted due to a very limited amount of sample obtained from the synthesis. II. Electrochemical Studies of the Complexes One of the main objectives of this project is to extend the electrochemical studies conducted on the (μ4-O)(denc)4Cu4-x(Ni(H2O))xCl6 [x = 0 to 4] complexes to other types of heteropolymetallic complexes (μ4-O)(denc)4M'4-xMxCl6 [M' and M = Cu, Zn, or Co, and x = 0 to 4]. In this work, we have shown that Cu3Co complex is electrochemically active and at higher cathodic potential the complex forms a deposit on the electrode surface. To acquaint the students with the electrochemical studies, we also conducted previous cyclic voltammetric (CV) studies on the Cu4 and Cu2Ni2 complexes.6 All CV studies were carried using PAR Model 263A Potentiostat – Galvanostat (PerkinElmer) with three electrodes system consisting of Pt working (3 mm diameter, CH instrument Co.), Pt wire counter, and Ag/(0.01 M) silver hexafluorophosphate (AgPF6) -CH3CN reference electrodes. The Pt working electrode was polished with 0.05 μm alumina (Buehler), washed with deionized water, sonicated for about 5 minutes, and dried before use. All experiments were carried out using 1 mM solutions of the complexes with 0.20 M tetrabutylammonium hexafluorophosphate as the supporting electrolyte in dimethylsulfoxide (DMSO). The solutions were bubbled with N2 prior to the electrochemical measurement and blanketed with N2 while conducting the experiments. CV studies as a function of switching potentials and pause time were conducted with the first scan after polishing the electrode while the rest of the scans were continuous. References 1. Davies, G.; El-Sayed, M. A.; El-Toukhy, A., Chem. Soc. Rev., 1992, 101. 2. Caulton, K. G.; Davies, G.; Holt, E. M., Polyhedron, 1990, 9, 2319. 3. El-Toukhy, A.; Cai, G. Z.; Davies, G.; Gilbert, T.; Onan, K.; Veidis, M., J. Amer. Chem. Soc., 1984, 106, 4596. 4. Dieck, H. T., Inorg. Chim. Acta, 1973, 7, 397. 5. El-Toukhy, A.; El-Essawi, M.; Tawfik, M.; El-Sayed, L.; Iskander, M. F., Trans. Met. Chem., 1982, 7, 158. 6. Workie, B.; Dube, C. E.; Aksu, L.; Kounaves, S. P.; Robbat, A. Jr.; Davies, G., J. Chem. Soc., (Dalton Trans.), 1997, 10, 1739 and refs therein. Figure 1. The effect of switching potential on CV of 1 mM of the Cu3Co core complex in 0.20 M TBAPF6-DMSO at a scan rate of 0.02 Vs-1. Figure 2. CV of 1 mM Cu3Co core complex in 0.20 M TBAPF6-DMSO at a scan rate of 0.02 Vs-1. Figure 3. CVs of 1 mM Cu3Co core complex in 0.20 M TBAPF6-DMSO at a scan rate of 0.02 Vs-1. Increasing peak currents correspond with pause time of 0, 10, 20, 40 and 100 s.