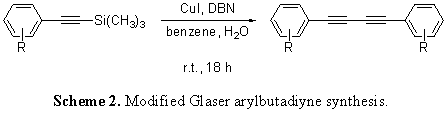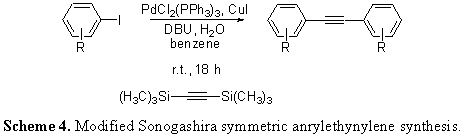ACS PRF | ACS
All e-Annual Reports

42984-GB1
Improved Synthesis of Arylethynylenes and Arylbutadiynes Utilizing an in Situ Ethynylsilane Deprotection Reaction
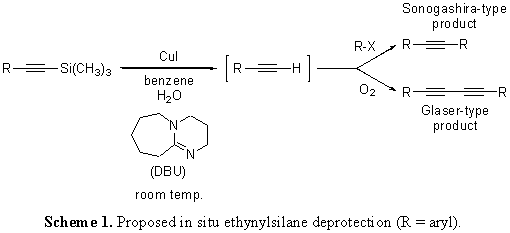
I. Original Aims The properties of arylethynylene and arylbutadiyne substructures make these moieties the building blocks of choice when studying shape-persistent supramolecular and nanoscale systems. By virtue of their structural rigidity, conjugation and unique bond torsion, sequence-specific arylethynylene and arylbutadiyne oligomers have become prevalent in many fields. Originally, we proposed a new synthetic methodology for the generation of such oligomers utilizing an innovative ethynylsilane deprotection. The reaction in question employs copper(I)-, amidine base- and water-dependant conditions to effect an ethynylsilane deprotection. Proposed experiments have investigated our hypothesis that the deprotection is amenable to additional coupling reactions in the same pot (Scheme 1).
The long term goal of the proposed work was improving the generation of oligomeric arylethynylene and arylbutadiyne architectures to allow for the rapid prototyping of a broad variety of important molecules. The specific aims that follow illustrate the proposed experiments for a two-year funding period: Original Aim 1 – Optimization of a Glaser-type ethynyl homocoupling protocol. Original Aim 2 – Implementation of the Glaser-type protocol in a study to probe its tolerance for substrates of varying steric and electronic substitution. Original Aim 3 – Confirmation of the utility of the new reaction protocol through applications in macrocyclization and oligomerization. II. Modified Aims Since the inception of the proposal originally sent to the ACS PRF, which highlighted an emphasis on the Glaser homocoupling usage for the in situ ethynylsilane deprotection, several new avenues of application have arisen. These modified aims, as well as the original, are listed below: Modified Aim 1 – Glaser Homocoupling: We are still investigating the applicability of the in situ ethynylsilane deprotection to Glaser-style generation of butadiynes. Initial work showed that the addition of dioxygen gas, as originally proposed, to the reaction mixture did not supply the desired product. In fact, all reactions attempted with O2 have lead to either reduced yields or difficult-to-characterize, polymeric products. Since that time, we have employed DBU's amidine counterpart DBN in reactions excluding purposeful dioxygen to generate butadiyne products in modest yield (Scheme 2). Currently, we are perfecting this strategy for application to oligomerization. Modified Aim 2 – Ethynylsilane Deprotection: Since the beginning of the funding period, a part of our group has “gone back to the drawing board” on the ethynylsilane deprotection reaction itself and had great success characterizing the method. After a short protocol study that resulted in the data shown in Scheme 3, a structural study was embarked upon that gave most products in quantitative yield. We are currently writing these data into a manuscript for publication in the journal Tetrahedron Letters. Modified Aim 3 – Modified Sonogashira with BisTMSA: With regard to previous findings, we found that the modified Sonogashira reaction that generated symmetrical bisarylethynylenes using trimethylsilylacetylene could also be affected using bis(trimethylsilyl)acetylene, further enhancing the efficacy of our methods. Using our previously published protocol (Mio, M. J.; Kopel, L. C.; *Braun, J. B.; *Gadzikwa, T. L.; *Hull, K. L.; Brisbois, R. G.; Markworth, C. J.; Grieco, P. A. Org. Lett. 2002, 4, 3199-3202.) we were able to show the method in Scheme 4 may have advantages to the original, including the use of solid BisTMSA as an alkyne synthon. After the completion of a structural study, we plan to write these data into a manuscript for the Journal of Organic Chemistry as a Note. Modified Aim 4 – Ethynylsilane Protecting Group Orthogonality: Finally, we have had success in the application of the in situ ethynylsilane deprotection with regard to synthetic orthogonality with other, bulkier ethynylsilanes. We are currently in the beginning stages of investigating whether appropriately-derived aryl rings can undergo our battery of reactions in the presence of other groups with future, separate deprotection in mind. With such a tool, the synthetic efficacy of our reactions will be greatly increased. III. Summary and Outlook The development and direct application of a new protocol for the generation of sequence-specific arylethynylene and arylbutadiyne oligomers has been proposed. In the short term, the strategies presented, which feature room temperature conditions and no supplementary reagents, are theorized to exhibit synthetic usefulness and flexibility. Ultimately, the advent of innovative approaches to the generation of these oligomers should allow for rapid molecular prototyping, and in turn, increase the accessibility of valuable supramolecular and nanoscale systems.