
ACS PRF | ACS
All e-Annual Reports

43176-AC4
In Search of Aryloxenium Ions
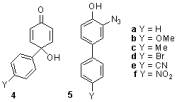
 Aryloxenium ions, 1, are invoked as intermediates in oxidation reactions of phenols, including many reactions that are synthetically or commercially useful.Since mechanistic studies were lacking, it was not clear that transient oxenium ions were formed in these reactions. Previously we reported that the ions 1a-d were generated by hydrolysis of 4-aryl-4-acetoxy-2,5-cyclohexadienones, 2, and O-aryl-N-methanesulfonylhydroxylamines, 3. The ions were indirectly detected by 18O-labeling studies in 18O-H2O, common ion effects, and the formation of the azide adducts 5 at the expense of the hydrolysis products 4 under conditions in which the decomposition rates of the precursors were independent of [N3-]. The ions 1e and 1f could not be detected by azide trapping during the hydrolysis of 2ex and 2fx even though 18O labeling shows that a fraction of the hydrolysis of both esters occurs through Calkyl-O bond cleavage. These esters exhibit only second-order trapping by N3-, although in both cases the characteristic adduct 5 is generated. We were also unsuccessful at generating the 4-Me substituted ion 1g under solvolysis conditions.
Aryloxenium ions, 1, are invoked as intermediates in oxidation reactions of phenols, including many reactions that are synthetically or commercially useful.Since mechanistic studies were lacking, it was not clear that transient oxenium ions were formed in these reactions. Previously we reported that the ions 1a-d were generated by hydrolysis of 4-aryl-4-acetoxy-2,5-cyclohexadienones, 2, and O-aryl-N-methanesulfonylhydroxylamines, 3. The ions were indirectly detected by 18O-labeling studies in 18O-H2O, common ion effects, and the formation of the azide adducts 5 at the expense of the hydrolysis products 4 under conditions in which the decomposition rates of the precursors were independent of [N3-]. The ions 1e and 1f could not be detected by azide trapping during the hydrolysis of 2ex and 2fx even though 18O labeling shows that a fraction of the hydrolysis of both esters occurs through Calkyl-O bond cleavage. These esters exhibit only second-order trapping by N3-, although in both cases the characteristic adduct 5 is generated. We were also unsuccessful at generating the 4-Me substituted ion 1g under solvolysis conditions.
During the current year we have investigated the possible photolytic generation of transient 1c from 2c and 3c. Steady state photolysis of 2c at 235-280 nm in pH 7.1 phosphate buffer generated 4c as a major product in yields of 30-35% after correction for photolytic decomposition. Irradiation of 2c in the presence of 40 mM N3- led to complete suppression of 4c. Since 5c is highly photoreactive we were not able to detect it. Photolysis of 3c leads only to 4x-methyl-4-biphenylol so 1c does not appear to be generated from this photolysis.
 Laser flash excitation of 2c was carried out at 266 nm in O2-saturated phosphate buffer. Two transient absorbance bands at λmax ca. 360 nm (A-360) , and λmax ca. 460 nm (A-460) were observed. A-460 decays with a first-order rate constant, ks, of (5.8 ± 0.4) × 106 s-1 that is independent of O2. The quenching of A-460 is N3--dependent with an apparently diffusion limited second-order rate constant, kaz, of (6.6 ± 0.2) × 109 M-1s-1.The intermediate detected at 460 nm is apparently identical with the indirectly detected ground-state intermediate 1c. The intermediate does not react with O2 indicating that it is not a radical, but it does react efficiently with the cation trap N3-.The ratio kaz/ks of (1.14 ± 0.09) × 103 M-1obtained from the LFP study is equivalent to the one obtained from the N3--trapping of hydrolysis of 2c at 30 °C ((1.0 ± 0.2.) ×103 M-1). Finally, the steady-state photolysis shows that the same product, 4c, is generated during photolysis and hydrolysis of 2c, and this product can be suppressed by N3-.
Laser flash excitation of 2c was carried out at 266 nm in O2-saturated phosphate buffer. Two transient absorbance bands at λmax ca. 360 nm (A-360) , and λmax ca. 460 nm (A-460) were observed. A-460 decays with a first-order rate constant, ks, of (5.8 ± 0.4) × 106 s-1 that is independent of O2. The quenching of A-460 is N3--dependent with an apparently diffusion limited second-order rate constant, kaz, of (6.6 ± 0.2) × 109 M-1s-1.The intermediate detected at 460 nm is apparently identical with the indirectly detected ground-state intermediate 1c. The intermediate does not react with O2 indicating that it is not a radical, but it does react efficiently with the cation trap N3-.The ratio kaz/ks of (1.14 ± 0.09) × 103 M-1obtained from the LFP study is equivalent to the one obtained from the N3--trapping of hydrolysis of 2c at 30 °C ((1.0 ± 0.2.) ×103 M-1). Finally, the steady-state photolysis shows that the same product, 4c, is generated during photolysis and hydrolysis of 2c, and this product can be suppressed by N3-.
A-360 decays in a biphasic manner with two first-order rate constants. It is not clear whether there are two simultaneously formed or two sequentially formed intermediates. Neither rate constant depends on [N3-] or O2. The lifetimes of these intermediates (ca. 12 μs and 75 μs) are much longer than that of 1c (170 ns). We are currently pursuing the identity of these intermediates and attempting to generate other oxenium ions by laser flash photolysis. Generation of potential triplet state ions by triplet sensitized photolysis is also being investigated.
The quinol esters 2h, 2i and 2hx, and the sulfonamide 3i were investigated as possible precursors to 4-alkylaryloxenium ions, however, with one possible exception, they do not generate oxenium ions. Ester 2h predominately undergoes ordinary acid and base catalyzed ester hydrolysis. Ester 2i decomposes under both acidic and 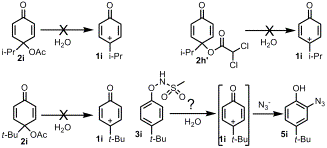 neutral conditions to generate tert-butanol and 1-acetyl-1,4-hydroquinone apparently by an SN1 mechanism. Decomposition of 2i in the presence of N3- leads to formation of the explosive 2,3,5,6-tetraazido-1,4-benzoquinone. Ester 2hx reacts with N3- to generate the two adducts 2-azido-4-isopropylphenol and 3-azido-4-isopropylphenol. Kinetic analysis shows that both adducts are produced by a kinetically bimolecular reaction of N3- with 2hx. Sulfonamide 3i predominately undergoes a rearrangement reaction under acidic and neutral conditions, but a minor component of the reaction yields 4-tert-butylcresol and 2-azido-4-tert-butylphenol, 5i, in the presence of N3-. These products may indicate that 3i generates the oxenium ion 1i, but they are generated in very low yields (ca. 10%) so it is not possible to definitively conclude that 1i has been produced. If 1i has been generated, the N3--trapping data indicate that it is a very short-lived and reactive species in H2O. Comparisons with similarly reactive nitrenium ions indicate that the lifetime of 1c is ca. 20-200 ps if it is generated, so it must react by a preassociation process. DFT calculations at the B3LYP/6-31G*//HF/6-31G* level coupled with kinetic correlations also indicate that the aqueous solution lifetimes of 1g-i are in the ps range.
neutral conditions to generate tert-butanol and 1-acetyl-1,4-hydroquinone apparently by an SN1 mechanism. Decomposition of 2i in the presence of N3- leads to formation of the explosive 2,3,5,6-tetraazido-1,4-benzoquinone. Ester 2hx reacts with N3- to generate the two adducts 2-azido-4-isopropylphenol and 3-azido-4-isopropylphenol. Kinetic analysis shows that both adducts are produced by a kinetically bimolecular reaction of N3- with 2hx. Sulfonamide 3i predominately undergoes a rearrangement reaction under acidic and neutral conditions, but a minor component of the reaction yields 4-tert-butylcresol and 2-azido-4-tert-butylphenol, 5i, in the presence of N3-. These products may indicate that 3i generates the oxenium ion 1i, but they are generated in very low yields (ca. 10%) so it is not possible to definitively conclude that 1i has been produced. If 1i has been generated, the N3--trapping data indicate that it is a very short-lived and reactive species in H2O. Comparisons with similarly reactive nitrenium ions indicate that the lifetime of 1c is ca. 20-200 ps if it is generated, so it must react by a preassociation process. DFT calculations at the B3LYP/6-31G*//HF/6-31G* level coupled with kinetic correlations also indicate that the aqueous solution lifetimes of 1g-i are in the ps range.