
ACS PRF | ACS
All e-Annual Reports

43535-AC5
Understanding Water and Organic Compound Oxidation at Boron Doped Diamond Film Electrodes
Introduction
The objective of this research was to investigate the effectiveness of boron doped diamond film (BDD) electrodes for removing contaminants from wastewaters. Work thus far has investigated oxidation of perfluorooctyl sulfonate (PFOS) and reduction of trichloroethylene (TCE).
Oxidation
PFOS was selected for investigating the effectiveness of BDD electrodes for contaminant oxidation because it is widely found in the environment and because perfluorinated compounds are the most difficult to oxidize of any class of organic compounds. The recalcitrance of PFOS to oxidation by advanced oxidation processes has been demonstrated in two studies attempting to oxidize PFOS using hydrogen peroxide with either Fenton's reagent, ultraviolet light or ozone ([1], [2]). In neither of theses studies was any oxidation of PFOS observed.
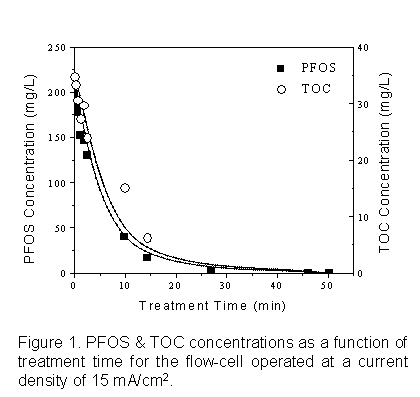
Figure 1 shows PFOS and total organic carbon (TOC) concentrations as a function of treatment time in a BDD flow-through reactor. Flow-cell reaction rates for PFOS and TOC were first order with respect to concentration, with a destruction half-life of ~7.5 minutes. Throughout the course of the experiments, no organic reaction products were detected and sulfate and fluoride concentrations were equivalent to 1 sulfate and 17 fluoride ions released per PFOS molecule degraded. This indicates that PFOS was completely mineralized in one interaction with the electrode surface.
Experiments investigating the mechanisms responsible for TCE reduction at BDD electrodes suggests that TCE chemically adsorbs at specific surface sites. Figure 2 shows the reaction rates for TCE as a function of the electrode potential. Reduction of TCE resulted in the stoichiometric production of acetate with no detectable intermediate products. This is very unusual since reduction of TCE by metal electrodes has been found to produce ethene and ethane ([3], [4]). TCE reduction at BDD cathodes was accompanied by a decline in the solution pH value, with an average of 4 moles of H+ produced per mole of TCE reacted. The absence of any intermediate chlorinated products suggests that TCE dechlorination occurred via a mechanism involving chemisorbed intermediates.
 |
This research demonstrated that PFOS can be readily oxidized at BDD electrodes. This is significant because all other methods tested have not been able to oxidize PFOS in aqueous solutions. This research was also the first to demonstrate that chemisorption reactions are likely involved in organic compound reduction at BDD electrodes. This finding opens up new possibilities for using BDD electrodes in the electrosynthesis of organic compounds.
 |