ACS PRF | ACS
All e-Annual Reports

43621-AC4
Probing Conjugation-Related Properties in Benzo- and Thieno-Fused Dehydroannulenes
The aim of this research program is to construct novel dehydrobenzoannulene (DBA) geometries and other annulene-like structures, and to investigate of the chemical reactivity and physical properties of the macrocycles to answer fundamental scientific questions. Specifically, this PRF-funded project is (1) studying the effect of 'global' transannular delocalization by the preparation of hybrids where a DBA ring is fused to [2.2]paracyclophane (PC), a moiety capable of through-space delocalization, and (2) exploring the conjugation properties and polymerization chemistry of a series of thieno-fused dehydroannulenes. The ultimate goal of our research program is the discovery of possible materials applications for this class of macrocycles. This will only come about after we obtain a better fundamental understanding of the DBA skeleton.
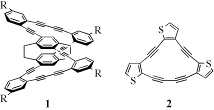
In the first area we prepared a series of [2.2]paracyclophane/dehydrobenzo[14]annulene hybrids (e.g., 1), [2.2]paracyclophane/dehydro[14]annulene hybrids and suitable model systems. Comparison of the electronic absorption spectra in each series of compounds illustrates conclusively the existence of global communication between the decks in the [2.2]paracyclophane unit. Delocalization over the entire hybrid structure is uniquely augmented since the appended annulene ring is locked into planarity. Our hybrids are proving useful for studying chromophore-chromophore interactions, as well as mimic through-space delocalization in conjugated organic materials, such as the closely related phenylene-ethynylenes. A full paper on this work recently appeared (Chem. Eur. J. 2006), along with a paper describing the use of the fused [2.2]paracyclophane as probes to measure annulene aromaticity (Org. Lett. 2005).
In the second area we have been incorporating thiophene moieties onto the dehydro[14]annulene skeleton. The choice of thiophene as the fused aromatic ring was inspired by factors such as the chemical and electrochemical polymerizability of thiophene, the ability to form two-dimensional p-systems useful for electronics and photonics, the easier polarizability of thiophene, and the interaction among the individual macrocycles due to the lone pairs on the sulfur in each thiophene ring. To date we have prepared a small 'library' (ca. 20) of dehydrothienoannulene (DTA) compounds (e.g., 2), which represent a large majority of the regioisomers resulting from thiophene fusion to the dehydro[14]annulene core. This study should allow firm interpretation of the subtle changes in electronic structure. A manuscript on this work has been submitted. The highly strained [14]DTAs also react with Na2S to generate planarized terthiophene derivatives in moderate yield. Comparison with their non-planarized counterparts is underway. A number of closely related [15]DTAs have also been prepared recently to examine the effects on the optoelectronic properties caused by disruption of overall macrocycle conjugation through inclusion of a cross-conjugated meta-arene linkage.