ACS PRF | ACS
All e-Annual Reports

43948-B1
Stereospecific Intramolecular Carbenoid Insertions on Furanose Platforms as a Route to Branched-Chain Sugars, C-Glycosides and Fused Heterocycles
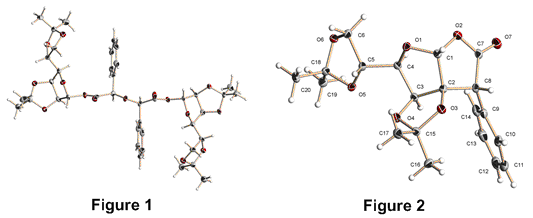
The use of enantiomerically pure carbohydrates for the synthesis of other complex molecules relies on the availability of methodology that is compatible with the associated functionality present in the saccharide substrate. To expand the available methods we are investigating the application of carbenoid insertion chemistry for the formation of carbon-carbon bonds on conformationally restricted furanose platforms. The method holds great promise for the stereospecific formation of chiral heterocycles, including those related to C-glycosides, and the frameworks of several classes on natural products. We have constructed a variety of furanose-derived diazoesters in which a singly-unprotected hydroxyl group from the sugar has been functionalized using typical ester-coupling conditions followed by diazo transfer from p-acetamidobenzenesulfonyl azide (p-ABSA) and base. Decomposition of these diazo compounds, using Rh2(OAc)4 in CH2Cl2, has led to very interesting results, including carbenoid insertion to form the predicted gamma-lactone, as well as side-products such as alpha-keto esters and alkenes, the products of carbenoid dimerization. Unexpectedly, we have also isolated dimeric ethers from these reactions in which two carbenoids have apparently reacted with a molecule of water. Interestingly, this reaction appears to be highly stereoselective and structures of the products have been proven by X-ray diffraction (see Figure 1). The stereochemical outcome of this process is under further investigation as a new route to enantiomerically pure ethers. The successful outcome of intramolecular C-H insertion at C-2 of a mannofuranose platform has likewise been proven by X-ray crystallography (Figure 2); however this compound has only been isolated in low yield and needs further attention. Our preliminary work with this route to sugar-derived diazoesters led to the discovery of a new one-pot synthesis of glycosyl azides from the corresponding lactol precursors and we have expanded the scope of this reaction to non-carbohydrate alcohols. The reaction involves displacement of azide from p-ABSA by the alcohol (or alkoxide), generation of an intermediate sulfonate ester, and subsequent SN2 reaction with azide anion to afford the corresponding azide. Initially, DBU was used as the base, in CH2Cl2 or CH3CN solution, but this led to only modest yields in the sugar chemistry. Changing the base to NaH, and the solvent to DMF, has improved yields greatly and we now have a truly one-pot conversion of alcohols to azides without needing to isolate a sulfonate ester intermediate. The method has been expanded to employ microwave heating, which shortens reaction times significantly. It has become apparent why the use of DBU leads to lower yields; DBU appears to react with p-ABSA when the two are heated in solution. The resulting generation of free azide anion offers an alternative to using sodium azide in SN2 reactions on alkyl halides and, indeed, this works very well to give a variety of non-carbohydrate azides. The reaction is amenable to the use of IR spectroscopy to monitor the fate of the different azide species involved (p-ABSA, free N3-, and the azide product), which is convenient especially for volatile alkyl halides.