ACS PRF | ACS
All e-Annual Reports

43041-B4
Synthesis, Metal Ion Complexation, and Emission Studies of Naphthalimide Based Fluoroionophores
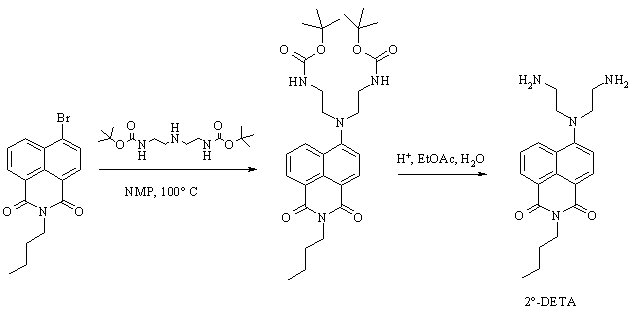
During this reporting period, the use of di-Boc protected diethylenetriamine was chosen as the method to synthesize the secondary amino substituted butyl naphthalimide (2°-DETA) followed by deprotection with dilute acid (schemes 1). This method works more consistently than other methods and we are currently optimizing the reaction and characterizing our products. Obtaining 2°-DETA will allow the completion of our planned studies of secondary ligand systems and macrocyclic ligand systems, having synthesized the cyclen and cyclam derivatives earlier this reporting period.
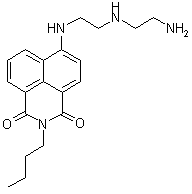
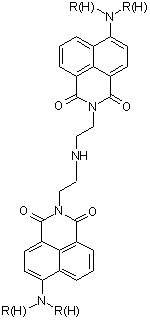
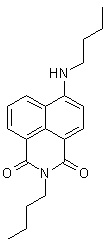
Scheme 1. Boc protected diethylenetriamine synthesis of 2°-DETA We have optimized the reaction to protect the primary nitrogens of diethylenetriamine and are currently working on optimizing the conditions to maximize the yield of the mono-protected triamine. The study and use of the Boc-protected polyamines for the synthesis of 2°-DETA fits well with the second target of our research group, described next. We have completed work on the acyclic systems we had planned with the study of 1°-DETA (figure 1). We have determined that addition of chain length and nitrogens to the ligand system does not increase metal ion selectivity or fluorescence enhancement. The next step is to probe the effect of the ligand system in the imide position. For this product, we require mono-Boc protected polyamines. We are synthesizing naphthalimides with the triamine in the imide position. Our initial reactions have produced coupling of two naphthalimides through the triamine linker (figure 2). We have synthesized small quantities of the diethylenetriamine imide product (figure 3) and are currently optimizing the reaction yield. We also plan to synthesize the ethylenediamine imide product and compare the photophysical properties with our previous results. Figure 1. 1°-DETA Figure 2. Coupling Product Figure 3. Diethylenetriamine imide product. We have begun a study of the photodegradation of 44 (figure 4). We are conducting the reactions in buffered water at different temperatures, pH's, and in the presence of different nucleophiles, irradiating with visible wavelengths to excite into the charge-transfer band of 44 in an attempt to understand the mechanisms of photoreaction and photodegradation. Our initial results following the reactions by GC-MS find the major product is 4-aminobutylnaphthalimide, suggesting an elimination mechanism in the excited state, presumably followed by addition to the alkene produced by advantages nucleophiles and rearrangement of the enolate form of the aminonaphthalimide. We are currently changing reaction conditions in an effort to detect the products of the presumed alkene product. Figure 4. 4-butylaminobutylnaphthalimide (44) During this reporting period, three students were directly supported by grant funds, three students were indirectly supported through materials purchased by grant funds, and projects advanced to the point that four additional students will be able to work on projects funded by this grant in the next reporting period. The research supported by this grant allowed these students to experience real research activities, work with equipment most undergraduate students do not have access to, and work toward becoming independent chemists, which will greatly aid them in there future endeavors. The undergraduate students working with me this reporting period are all graduate school bound, so research is an excellent activity to prepare them for their next phase of training. The research project funded during this reporting period has allowed me to continue my personal study in chemistry. Research activities are the primary means for academic chemists to remain current in the field. This in turn reflects in their teaching performance at all levels and their level of excitement for chemistry, which is definitely noticed by students.