ACS PRF | ACS
All e-Annual Reports

44453-AC1
Development of New Reactions Based on Decarboxylative Metalation
Aim: Develop asymmetric syntheses of a-fluoroketones and allylic selenides based on decarboxylative coupling.
Activity: Asymmetric synthesis of a-fluoroketones.
We have developed and reported a strategy for the enantioselective synthesis of a-fluoroketones. The general process involves the formation of fluoroenolates via catalytic decarboxylation and the control of their subsequent allylation using asymmetrically ligated catalysts. This is a particularly noteworthy development because fluoroenolates can not be generated by standard base-induced enolate formation. Thus, our ability to regiospecifically generate enolates is superior to conventional methods and allows access to enolates that previously were only accessible through difficult procedures.
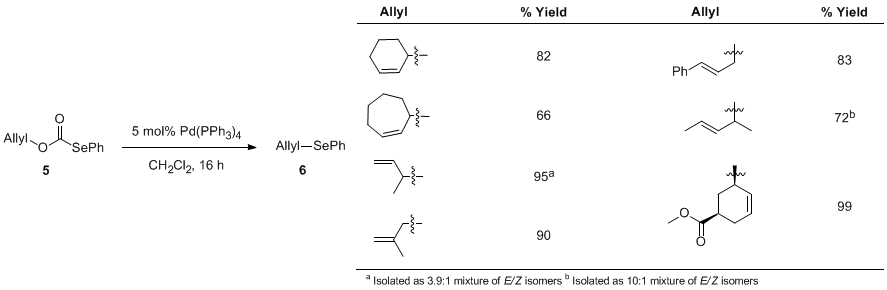
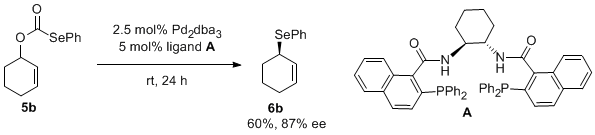
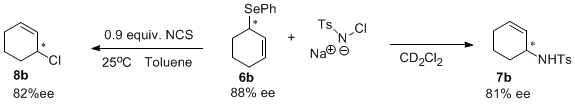
Activity: Decarboxylative coupling of allylic selenides. We have developed a straightforward route to allylic selenocarbonates like 5. Treatment of the selenoformate with catalytic Pd(PPh3)4 indeed provided the allyl selenide 6 in good yield. However, this was promising since other methods for palladium-catalyzed selenation failed to give any of this product. Our hypothesis is that, while nucleophilic selenides often poision palladium catalysts, the selenocarbonate selenium atom is less nucleophilic, thus the palladium catalyst does not readily poison. However, relatively high loadings (5-10 mol%) of the palladium catalyst are currently required. We have looked at the scope of this reaction and were excited to find that more highly substituted allyl alcohol derivatives are superior substrates. Once again, we have conducted a preliminary screening of ligands and have identified the naphthyl Trost ligand is the best ligand for this chemistry. It is also interesting to note that the highest ee's are obtained for reactions that proceed to <60% conversion. We are currently operating under the hypothesis that kinetic resolution of the allylic selenoformate is taking place. Having access to optically active allylic selenides has allowed us to investigate the sigmatropic rearrangement chemistry of such species. For example, allylic amines can be obtained by oxidation with chloramine T. However, the yield of this reaction is low (ca. 40%), so optimization of the reaction conditions is still necessary. Perhaps even more exciting, we have found that chlorination with NCS provides the allyl chlorides with high conservation of enantiomeric excess. This is noteworthy because we are unaware of any other method that can produce such highly enantioenriched allylic chlorides.