
ACS PRF | ACS
All e-Annual Reports

44202-AC1
Studies on the Mechanism of Enyne Metathesis
The collaborative ACS-PRF sponsored research is directed to the study of the enyne metathesis reaction mechanism. The enyne metathesis and the Grubbs carbenes that are commonly used are illustrated in Scheme 1. The primary research activity during year one was devoted to a full investigation of the ligand-promoted insertion for a variety of ruthenium carbene complexes and the development of a practical ruthenium carbene clean-up procedure for metathesis. This insertion was proposed as a way to stop metathesis reactions for kinetic analysis to study reaction mechanism, but it has evolved into a practical procedure to both stop a metathesis reaction and to facilitate purification of the organic products.
Scheme 1. Ene-yne Metathesis (eq 1), the Grubbs Carbenes and Intramolecular, Ligand-Promoted Carbene Insertion (eq 2)
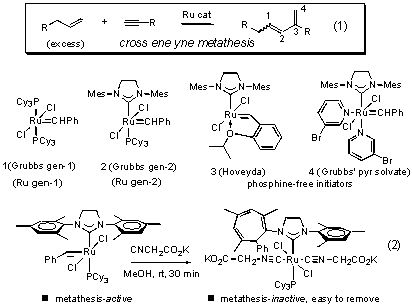
Carbene Insertion
We continued investigation into the carbon monoxide and isocyanide-promoted carbene insertion in several different Grubbs carbene complexes. The insertion chemistry was explored for a range of carbene substituents. We were interested to know how general the reaction of eq 2 was, so heteroatoms and vinyl substituents were investigated. We studied the range of ligands that could be used to promote the insertion in the second generation Grubbs carbenes to understand how ligand properties influence the reaction. We established the structures of several insertion products on the basis of spectral data and correlation with several crystal structures. The full study of various ligand-promoted substituted carbene insertions, including solid-state structural data and NMR studies is being completed and written up in a manuscript to be submitted in the next three months.
We have also studied the insertion in Grubbs complexes of different ligand environments, including (Cy3P)2Cl2RuCHR. The ligand-promoted deactivation of the first generation complex works, but the products are different from the cycloheptatrienes (eq 2) obtained through ring expansion in the second generation Grubbs carbenes.
Ruthenium Clean-Up Procedure
The carbene insertion led to both deactivation of the Grubbs carbene and transformation to a noncarbene coordination complex. We realized that these two characteristics fit nicely with our desire to stop metathesis reactions and also to remove the ruthenium at the end of the reaction. We synthesized a polar isocyanide and showed that the ligand resulted in rapid destruction of carbene, stopping catalysis and facilitated the removal of ruthenium(II), which we quantitated by inductively coupled plasma (ICP) spectrophotometry. These experiments showed that this was a ‘well-defined' transformation of the metal carbene and showed the effectiveness of the removal from several typical alkene and enyne metathesis applications. Because we felt that this would be generally helpful to the synthesis community, we invested time to study this for a variety of metathesis reactions and several different ruthenium(II) carbene complexes. This work has been published. We recruited a faculty co-author from Canisius College (a local four year school) to assist with the analytical work, and he appeared as co-author.
Mechanism of Ene-Yne Metathesis
Mechanistic studies have continued in this first year mostly in the insertion chemistry designed to stop enyne metathesis of internal alkyne substrates, where we could not use IR spectroscopy to follow the reaction. Thus the ligand-promoted insertion has been fully developed as a useful tool to stop metathesis. The kinetic studies will proceed in Year Two in internal alkynes and will investigate any rate differences between the first- and second generation carbenes. We will interpret these data in terms of our ongoing synthetic studies in ene-yne metathesis, and collaterally use this knowledge to develop new applications of the ene-yne metathesis.