
ACS PRF | ACS
All e-Annual Reports

42880-GB7
Well-Defined Initiators for the Synthesis of Biodegradable Polymers from Biorenewable Resources
Introduction: Lactide polymerization continues to be actively researched as illustrated, in part, by the many PRF-funded grants which include lactide polymerization proposals. Lactide monomer biorenewability, of as well as the biodegradability of poly(lactic acid), drives continued interest. We have proposed the synthesis of putative lactide polymerization initiators. During the funding period, we have made progress on the original proposed synthesis as well as beginning a new but related line of initiator synthesis.
 Pentadenate Mannich Route: Our initial PRF funded research developed reliable protocols for the synthesis of high purity 2-(2-(alkylamino)ethlamino)ethanols (1). A variety of these compounds have be isolated as the bishydrochloride salts in >10 g quantities. There are many literature examples of N,N'-dialkylethylenediamine compounds undergoing Mannich condensations with 2,4-dialkylphenols. The products of these reactions are invariably reported to precipitate from refluxing alcohol. When compounds 1 were subjected to Mannich conditions, no precipitation from the reaction was observed despite variation of solvents, reaction temperatures, substrate ratios, nature of R and R', and pH. Simple organic work-ups and standard methods to encourage product precipitation were unsuccessful. Ultimately, flash chromatography was effectively employed to purify and isolate the desired compounds (2). A representative 1H-NMR for 2 is shown below. The molecule's unsymmetrical structure results in non-first order couplings, although 2 is symmetric enough that some chemically-inequivalent resonances overlap and almost appear equivalent (e.g. aryl protons a). Metalation of 2 is currently under investigation, as originally proposed.
Pentadenate Mannich Route: Our initial PRF funded research developed reliable protocols for the synthesis of high purity 2-(2-(alkylamino)ethlamino)ethanols (1). A variety of these compounds have be isolated as the bishydrochloride salts in >10 g quantities. There are many literature examples of N,N'-dialkylethylenediamine compounds undergoing Mannich condensations with 2,4-dialkylphenols. The products of these reactions are invariably reported to precipitate from refluxing alcohol. When compounds 1 were subjected to Mannich conditions, no precipitation from the reaction was observed despite variation of solvents, reaction temperatures, substrate ratios, nature of R and R', and pH. Simple organic work-ups and standard methods to encourage product precipitation were unsuccessful. Ultimately, flash chromatography was effectively employed to purify and isolate the desired compounds (2). A representative 1H-NMR for 2 is shown below. The molecule's unsymmetrical structure results in non-first order couplings, although 2 is symmetric enough that some chemically-inequivalent resonances overlap and almost appear equivalent (e.g. aryl protons a). Metalation of 2 is currently under investigation, as originally proposed.

Indolyl Scorpionate Route: Polyindolylmethanes have long been know and, in work pioneered by Mason, have recently been used as ligands for main group and transition elements. Indoles undergo condensation reactions with aldehydes in the presence of catalytic acid to form diindolylmethanes. Unsubstituted indoles react preferentially at C3, resulting in compounds where the potentially coordinating nitrogens are pointed away from each other. When the indole C3 position is blocked by substitution, reactivity is forced to C2, yielding potentially chelating 3. The simplest C3-substituted indole, 3-methylindole, is conveniently inexpensive.
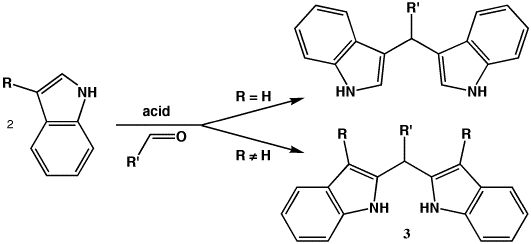 When R' includes a hydroxyl, 3 is analogues to 2 as both are trivalent with an alcohol which could initiate polymerization. The commercial availability and low cost of salicylaldehydes make them attractive starting points for investigation of the hydroxy[alkyl/aryl]di(3-methylindolyl)methane architecture. Though the phenoxy chelate would be a seven-membered ring, they have precedent with aluminum and ligands such as binapthalenediol (BINOL), binapthalenediamine (BINAM) and succinic acid, among others. Transition metals and lanthanides also form seven-membered chelates.
When R' includes a hydroxyl, 3 is analogues to 2 as both are trivalent with an alcohol which could initiate polymerization. The commercial availability and low cost of salicylaldehydes make them attractive starting points for investigation of the hydroxy[alkyl/aryl]di(3-methylindolyl)methane architecture. Though the phenoxy chelate would be a seven-membered ring, they have precedent with aluminum and ligands such as binapthalenediol (BINOL), binapthalenediamine (BINAM) and succinic acid, among others. Transition metals and lanthanides also form seven-membered chelates.
We have prepared several hydroxyphenyldi(3-methylindolyl)methanes. Typically, 3-methylindole and the salicylaldehyde were refluxed in alcohol in the presence of catalytic acid. Off-white precipitate began forming within an hour and reaction progress was monitored by TLC; excessive reaction times resulted in product degradation and reduced yields. Crude product is easily isolated by vacuum filtration and recrystallization yields feathery white crystals in unoptimized yields of 65-91%. Melting points for these compounds are uniformly high (>208 °C). While some melting point ranges are narrow (3 °C), hygroscopicity caused broadening of others (6 °C). These compounds display expected NMR spectra (indexed below). Assignments of the NH and OH resonances was supported by exchange with added D2O. Also, hydroxymethyldi(3-methylindolyl)methane (R' = CH2OH) was prepared in two steps from allyl acetate. This derivative has a more flexible putative initiating group which could form a six-membered chelate.
Neither metalated tri(3-methylindolyl)methanes (3, R = 3-methylindol-2-yl), nor metalated tridentate di(3-methylindolyl)methanes with pendent heteroatoms have been reported. There is one tri(3-methylindolyl)methane bonded to phosphorus through the indole nitrogens, as metal chelation might be expected to occur. Thus, it is perhaps unsurprising that initial metalation attempts have not yielded the desired compounds. Alternate routes are under investigation.
NMR of 3: (R = Me, R' = 2-hydroxyphenyl) 1H NMR (DMSO, 300 MHz) d 10.29 (s, 2H), 9.55 (s,1H), 7.39 (d, 2H), 7.29 (d, 2H), 7.07 (d, 2H), 6.96 (m, 4H), 6.85 (d, 1H), 6.76 (t, 1H), d 6.21 (s, 1H), d 2.00 (s, 6H). 13C NMR (DMSO, 75 MHz): d 154.32, 135.32, 134.68, 129.53, 128.82, 127.67, 127.19, 120.20, 119.01, 118.04, 117.51, 115.04, 111.03, 105.89, 34.76, 8.29.
Student Impact: During the reporting period, summer student researchers obtained competitive internal funding for stipends and thus no salaries were paid from the grant. Research supplies purchased from the grant supported four undergraduates during this period. An ozone generator purchased from grant funds was key to successful completion of one synthesis. The students involved in these projects were already or have become chemistry or biochemistry majors and have experienced positive outcomes due to participation. One graduated in May of 2007 with high honors (a research thesis based designation), one received the national Iota Sigma Pi Gladys Anderson Emerson award and one participated in Cornell's REU program this past summer. One student, a senior chemistry/physics double major, plans to pursue doctoral studies in the physical sciences.