ACS PRF | ACS
All e-Annual Reports

41897-B10,4
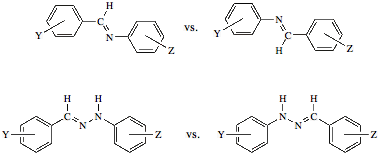
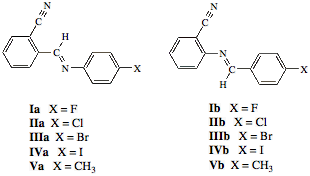
The Preparation and Characterization of Novel Organic Solids
Scheme 1. "Bridge-flipped" isomeric benzylideneanilines (upper) and phenylhydrazones (lower). In our first two research seasons, we prepared and determined the crystal structures of a variety of benzylideneanilines and phenylhydrazones and identified three new isostructural pairs, effectively doubling the number of known examples. During this past research season, we have determined the crystal structures of pairs of bridge-flipped benzylideneanilines that might engage in intermolecular Lewis acid-Lewis base contacts that, if present in both crystalline isomers, would favor the formation of isostructural crystals. In the crystallographic literature can be found numerous examples of crystal structures of molecules bearing both an iodine atom and a nitrogen atom (in a nitrile group or in a heterocyclic ring) in which the packing arrangement involves a close Lewis acid-Lewis base intermolecular interaction between these atoms. Molecules in these crystals are organized into chains in the solid state by means of this close intermolecular approach. Recently we have examined benzylideneanilines bearing both a halogen atom and a nitrile group by single-crystal X-ray diffraction to determine whether close halogen-nitrile approaches in the individual bridge-flipped isomers might organize the molecules into similar solid-state packing motifs (such as chains of molecules) that might encourage isostructuralism. As part of this effort, we have recently determined the crystal structures of a series of four pairs of isomeric benzylideneanilines bearing a halogen atom at a para position and a nitrile substituent at an ortho position; we have also examined the methyl analogues (Scheme 2). Scheme 2. Bridge-flipped pairs of halogen/nitrile-substituted benzylideneanilines (and methyl analogues) for which crystal structures have been determined in this work. We have found that none of the bridge-flipped isomeric pairs in this series form isostructural crystals, although isostructuralism does occur within both the cyanobenzylidene series (IIa and IIIa are isostructural) and the cyanoaniline series (Ib, IIb, and IIIb are isostructural). Given the relative strengths of their halogen atoms as Lewis acids, the fluoro compounds would be least likely to engage in intermolecular halogen-nitrile contacts in the solid state; the iodo compounds would be most likely to engage in such interactions. In accord with this prediction, none of the fluoro, chloro, or bromo isomers exhibit intermolecular halogen-nitrile interactions, and in their absence, different packing arrangements are assumed by the bridge-flipped isomers. The two iodo isomers, in striking contrast, do exhibit halogen-nitrile intermolecular contacts, yet these are not sufficient to compel the two compounds to assume similar packing arrangements. The methyl isomers assume crystal structures different from those of any of the halogenated analogues, indicating that the effect of the halogens on the packing is more than simply steric. My undergraduate students presented posters on this work at the spring 2007 national ACS meeting and are co-authors of my two recent publications; I presented a lecture on this work at the 2007 national meeting of the American Crystallographic Association.