ACS PRF | ACS
All e-Annual Reports

42320-B1
Approaches to the Synthesis of C-Linked Glycosyl Amino Acids
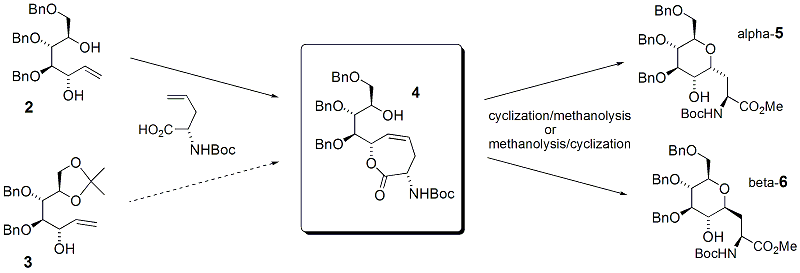
The biochemical understanding of protein glycosylation is making great strides, and interest is expanding in developing a variety of glycoconjugates and their mimics as enzyme inhibitors and as pharmaceutical targets. A simple, economic method for constructing metabolically stable isosteres of O-glycosyl amino acids or peptides would be highly beneficial. Our focus entails altering the nature of the connecting chain between the glycoside and the peptide through a methylene-for-oxygen substitution, namely the formation of C-glycosyl amino acids, such as 1. C-linked glycosides are robust to degradation by glycosidases, reaction with glycosyltransferases, acid hydrolysis of the former anomeric acetal, and -elimination from the serine, and thus offer a platform from which to explore their biochemical impact and pharmaceutical potential. Although initial advances were described with a cross-metathesis strategy for the preparation of compounds like 1, we have found advantage in using a ring-closing metathesis (RCM) strategy. Toward that end, a common intermediate 4 has been prepared from compounds 2 or 3. Rapid entry into this pathway was provided through the glycol-heptene 2, and conversion to 4 was achieved by esterification and RCM. The esterification step is unfortunately not selective for the allylic, secondary hydroxyl over the other secondary hydroxyl. Therefore, compound 3, derived inexpensively from mannitol, has become the preferred route. All steps from mannitol to 4 are complete except a final benzylation of a primary hydroxyl in the presence of a secondary hydroxyl. With the common intermediate 4 in hand, we have demonstrated that electrophilic cyclization onto the alkene, followed by methanolysis affords the alpha-isomer 5, whereas a reversal of these two reactions yields the beta-isomer 6.