ACS PRF | ACS
All e-Annual Reports

44401-G1
Investigations into the Reactivity of Vinyl Trichloromethyl Carbinols in the Jocic Reaction
Few general processes exist for the one-carbon oxidative homologation of aldehydes to carboxylic acids. Reported methods require special reagents or complex multi-step procedures and all are limited in scope, especially regarding homologation of enals or sensitive chiral substrates. We have devised a two-step homologation of aldehydes to one-carbon extended carboxylic acids by way of trichloromethyl carbinols in a Jocic-type reaction. Treatment of a carbinol with sodium borohydride or in situ generated sodium phenylseleno(triethyl)borate in a basic alcohol solvent generates the homologated carboxylic acid in high yields (Scheme 1).
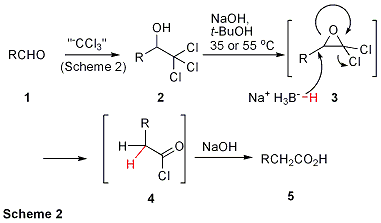
Recognizing the potential for the reactive gem-dichloroepoxide intermediate (3) formed during a Jocic-type reaction to undergo reductive ring opening via a hydride delivery agent, we considered conditions suitable for the process (Scheme 2). The protic solvent required to form 3 from 1 limited our selection of viable hydride sources. Sodium borohydride was particularly attractive due to its affordability, ease of handling, and stability in basic protic media. Given the tolerance of our designed reaction to moisture, standard tert-butanol served as a suitable medium for hydride delivery/acylation while precluding epoxide solvolysis. Treatment of both aliphatic and aromatic trichloromethyl carbinols with NaOH and NaBH4 in tert-butyl alcohol afforded one-carbon extended carboxylic acids in uniformly high yields. Although the oxidative homologation of aliphatic and aryl aldehydes worked well using Method A (Scheme 1), trichloromethylated enals afforded several products under corresponding conditions. Results from our investigation into the regioselective substitution of alkenyl gem-dichloroepoxides with various nucleophiles suggested a possible solution to these complications with enals. We recognized that a polarizable nucleophile, such as a selenide, would provide superior regioselectivity via an SN2 pathway in the addition to 3. The phenylseleno(triethyl)borate complex, easily prepared by mixing diphenyldiselenide and NaBH4 in EtOH, worked beautifully in the regioselective opening of alkenyl gem-dichloroepoxides (Scheme 3). This alternative method (Method B) also featured slightly improved results in the oxidative homologation of aromatic aldehydes relative to Method A. A plausible mechanism for conversion of allylic or aryl trichloromethyl carbinols to the desired acids is shown in Scheme 3. Support for this mechanism includes the appearance of 8 as an isolable intermediate and complete recovery of the diphenyldiselenide after work up. This latter fact allows for reuse of the diphenyldiselenide in subsequent homologations with no decrease in reaction efficiency. Hence, this approach, like that employing NaBH4 in t-BuOH, is remarkably economical. The requisite trichloromethyl carbinols were easily obtained by the method of Corey and Link. The buffered solution of trichloroacetic acid and sodium trichloroacetate in DMF allows trichloromethylation to occur without aldehyde enolization. Thus, the overall homologation process is compatible with aldehydes bearing a-chirality centers. The affordability and commercial availability of the reagents, innocuous by-products generated, operational simplicity, broad substrate scope including enals and enolizable aldehydes, and uniformly high yields make this protocol superior to alternative homologation procedures. The gracious contribution from the Donors to the Petroleum Research Fund has made this research possible. Without such support, the training and output of the two graduate students conducting these projects would be dramatically minimized, thereby jeopardizing their potential to obtain desirable post-graduate career or educational opportunities. My scientific output as a tenure-track assistant professor would also be delinquent in terms of University expectations for retention and promotion. Thus, the ACS PRF award has been a godsend for our research program and the continuance of the long-term career objectives of my students and me.