ACS PRF | ACS
All e-Annual Reports

44682-G4
Functional Zippers to Organize Synthetic and Biological Polymers
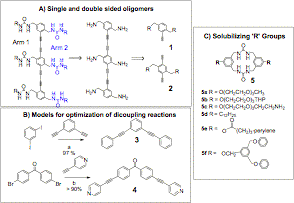
Specific aims: Nature makes assemblies of discrete size and shape that selectively catalyze reactions, recognize molecules with high specificity, and direct translocation. Our proposal targets the synthesis of discrete finite structures using urea-urea based zippers as an organizational tool. It is easy to make infinite extended structures with ureas. The challenge is to use this motif to form well-defined assemblies of finite size and shape. Higher order (tertiary and quaternary) structures may dramatically affect synthetic polymer properties, potentially extending their applications and tuning their specificity. We focused on the synthesis of rigid rod phenyl bisacetylenes that are preorganized to self assemble into dimers, trimers in solution (Figure 1). In this first year of the grant we have advanced our research on two fronts: 1) Optimization of the Sonogashira-Hagihara coupling reactions to generate of the rigid phenyl bis-acetylenes and 2) Establishment of the solution state assembly of bis-ureas including quantification of the monomer association constants.
Figure 1: Functional zippers bring together separate polymer chains to form discrete mesoscale assemblies Results: In this first reporting period we targeted the synthesis of single and double-sided oligomers that are programmed to assemble into well-defined structures through strong urea hydrogen bonds (Scheme 1A). The single sided models are predicted to self-associate into discrete dimers while the double-sided models with four or six urea groups can self-associate using the 'twisted' urea orientations to generate complex architectures. The monoalkyne building block 1 was readily synthesized; however, the dialkyne 2 proved challenging. To gain expertise in utilizing Sonogashira-Hagihara coupling conditions for aryl diiodides and dibromides, we used the model compounds 3 and 4 in Scheme 2. Recently, we successfully coupled both models isolating the dialkynes in high yields (> 90%). These models can also yield assembled structures, for example 4 forms a soluble spherical cage upon addition of Pd 2+. Scheme 1: Oligomeric ureas form defined assemblies in solution. (A) Single sided (containing one arm) and double sided (two armed) trimers can be synthesized from building blocks 1 and 2. (B) Two models 3 and 4 were used to optimize the coupling conditions. Reagents and conditions a: Pd(PPh3)2Cl2 (10 mol %), CuI, Piperidine, 0 °C, 3h, N2 (g); b: Pd(PPh3)4 CuI, K2CO3, DME/H20 (10:7), N2 (g), H2(g), 60 °C 20 h. (C) Investigation of solubilizing 'R' groups using bis-urea macrocycle 5. We evaluated a series of R groups to determine what group would best solubilize the ureas without hindering the urea self-association. Test macrocycles 5 was used to investigate solubilizing groups as it is readily synthesized yet insoluble. Long chain alkanes, including hexyl, dodecyl or octadecyl did not increase the solubility of the macrocycle 5. In contrast the polyethylene glycol derivatives 5a-c showed a marked increase in solubility, displaying limited solubility even in dichloromethane and chloroform. NMR dilution experiments in TCE show that the PEG derivative 5a self-associated; however, it appears that an internal hydrogen bond exists between one of the ethylene glycol oxygens and a urea NH at dilute concentrations. PGSE NMR experiments suggest that 5a dimerizes in TCE. The derivative 5f that contains a Frechet dendron also displays increased solubility and is unlikely to have competing intramolecular hydrogen bonds. We are studying its self-association by 1H NMR and IR. These experiments provided our first experience in analyzing supramolecular complexes in solution. We have determined binding constants and are now set to employ these new skills to analyze the assembly of the open chain urea oligomers. Goals for the next year. In the next grant period we will apply our new expertise with Sonogashira-Hagihara coupling reactions to prepare both the single and double sided building block 2. The acetylenes of 1 and 2 will be connected using Cu(OAc)2 to form the bis-acetylenes. Varying the stoichiometry of 1 and 2 should produce a range of different size oligomers that will be readily separable. Once the oligomers are purified and characterized the ureas will be generated using commercial or readily made substituted isocyanates. From our studies on model compound 5 we will use the branched ethylhexl chain or the Frechet dendron in 5f as the solubilizing substituent. We will study the structure of these oligomers in solution by 1H NMR, taking spectra at varying oligomer concentrations. These studies will provide guidelines for the design of linear polymers that are preorganized to fold to discrete tertiary structures.