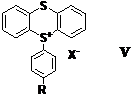ACS PRF | ACS
All e-Annual Reports

42557-AC4
Design and Synthesis of Novel Sulfonium Salt Photoacid Generators
Triarylsulfonium salts comprise the most widely employed class of photoacid generators that are employed in the areas of advanced microelectronic photoresists, stereolithography, electronic encapsulation, coatings, adhesives and printing inks. While these compounds possess inherent short wavelength UV sensitivity, they are essentially insensitive to wavelengths in the region 300-400 nm. At the same time, the number of potential applications for long wavelength absorbing photoacid generators continues to multiply rapidly. This is the second report describing the results of a PRF sponsored work designed to synthesize triarylsulfonium salts that possess photosensitivity in the 300-400 nm region.
Using the straightforward synthetic method shown in equation 1, a new series of triarylsulfonium salt photoacid generators with structures I-IV were prepared. The yields of triarylsulfonium salts using this method range from 63-94%. Purification was achieved by recrystallization from isopropanol. All of the sulfonium salts shown were observed to be photoactive and to undergo photolytic cleavage of a carbon-sulfur bond when irradiated with UV light.
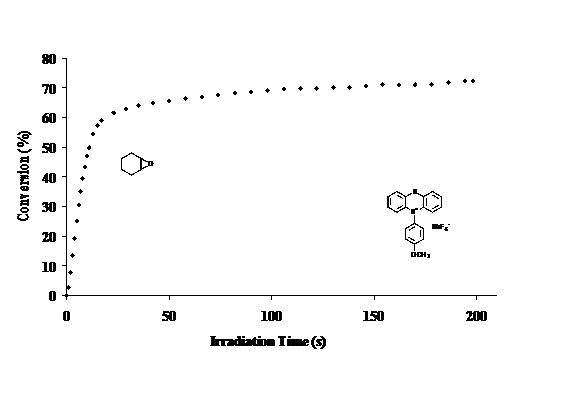
I II III IV As shown above, these photoacid generators were synthesized by condensing the readily available diphenyl sulfoxide with the corresponding thioxanthone compoundin the presence of a mixture of methanesulfonic acid and P2O5 (Eaton's reagent). The above photoacid generators have photoactive absorption bands lying in the 330-380 nm spectral region. As a result, they function well at the 313 and 365 nm bands emitted by mercury arc lamps. Thianthrene is a readily available heterocyclic sulfur compound. Oxidation of thianthrene with dilute nitric acid yields thianthrene-S-oxide. Subjecting this latter compound to Eaton's Reagent in the presence of a suitable activated aromatic compound yielded a series of heterocyclic triarylsulfonium salts with the general structure V. The product isolated in this reaction is invariably the para-substituted one as shown. Ortho products, if formed, must be produced in very small amounts. Typically, X- is an anion of low nucleophilicity and R can be varied widely. In most cases, R is an activating group such as, alkyl or alkoxy. The above compounds are crystalline salts that are readily purified by recrystallization. By changing the length of the R group, the solubility of this series of thianthrenium salts can be readily modified. This class of photoacid generators have absorption bands in the targeted region (300-320 nm). The dominant UV absorbing chromophor appears to be the heterocyclic sulfonium salt group since modifications of the R group do not bring about significant shifts in the lmax of these compounds. Thianthrenium salts display excellent photoinitiator characteristics for the ring-opening polymerization of epoxide monomers. This can be seen in Figure 1 in which the photopolymerization of cyclohexene oxide was carried out. Figure 1. Study of the photopolymerization of cyclohexene oxide carried out in the presence of 5(4-methoxyphenyl)thianthrenium hexafluoroantimonate (0.5 mol%). (light intensity 128 mJ/cm2) While we have not conducted experiments to determine the mechanism of the photolysis reaction of thianthrenium salts, the pathway for these compounds is presumed to be the same as for triarylsulfonium salts. This involves the photoinduced cleavage of a carbon-sulfur bond of the sulfonium moiety. In addition, to their apparent high quantum yields for photolysis, these compounds also display excellent thermal stability. For this reason, they do not spontaneously thermally induce the polymerization of the most reactive cationically polymerizable monomers even on prolonged storage at temperatures up to 100-120 oC. This makes these photoacid generators attractive for imaging and photoresist applications where pre- and postbake processes are carried out. The current project has been highly instructive for the graduate and postdoctoral coworker involved in its completion. They have had hands-on experience in photochemical reactions and in the use of highly specialized equipment employed in photopolymerizations. They also have been engaged in the evaluation of these photoinitiators in photoresist formulations, 3-dimensional imaging and in graphic arts imaging. Some of the results produced during this project have already been presented at recent meetings and conferences. Manuscripts are in preparation to report the details of the work.