ACS PRF | ACS
All e-Annual Reports

45277-B1
Investigation of the Butyllithium-Induced Sigmatropic Rearrangement: Intramolecular Cyclization Reaction of Allyl-Dichlorovinyl Ethers
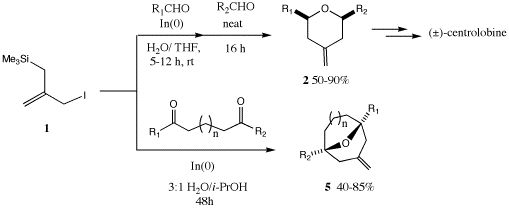
A major goal of our research program is to develop environmentally benign methods for carbon-carbon bond-formation for use in natural products synthesis. Transformations in which multiple bonds are made in a single reaction are of special interest in this context. With the funding we have enjoyed from the ACS Petroleum Research Fund this past year, we have been able to make significant progress along these lines in a number of important areas. Our original objective of being able to efficiently prepare seven and eight membered carbocycles in a benign fashion has been realized in a remarkably simple [m+n] annulation process involving the combination of 3-iodo-2-[(trimethylsilyl)methyl]propene and dicarbonyl compounds in water in the presence of indium metal. This two-step, one-pot reaction involves intermolecular indium allylation of one carbonyl of the substrate, followed by indium-iodide (generated in situ) promoted intramolecular allylsilane cyclization. Via the formation of two carbon-carbon bonds and a ring, diverse oxa-bridged 7- and 8-membered carbocycles are produced in good yields in this process, and the procedure offers a truly "environmentally friendly" alternative to the analogous tin-mediated annulation reaction. We have also found that this reaction can be extended to the preparation of 2,6-disubstituted tetrahydropyrans, moieties found in numerous natural products of medicinal import. We have successfully utilized this approach to accomplish a formal synthesis of the natural product (±)-centrolobine, which is the shortest synthesis of this substance to date.
We have also developed an improved procedure for the formation of allyl alkynyl ethers, which undergo rapid low-temperature sigmatropic rearrangement to furnish g,d-unsaturated esters. The reaction of allylic alcohols and a-diazoketones in the presence of indium triflate as catalyst rapidly furnishes a-alkoxyketones. Enol triflates derived from undergo a facile E2 elimination at Ð78°C when treated with potassium tert-butoxide to furnish allyl alkynyl ethers, which immediately undergo sigmatropic rearrangement and trapping with an alcohol to furnish the unsaturated ester products. This process avoids numerous problems encountered with our previously developed method proceeding from allyl-1,1-dichlorovinyl ethers, which required the use of toxic CCl4 for precursor generation and the nucleophilic base butyllithium for rearrangement. It is anticipated that this rearrangement protocol will be very useful in the synthesis of natural products, and we are currently investigating a tandem rearrangement/cyclization reaction of benzyl alkynyl ethers for the preparation of indanones, which may be applied to a synthesis of (±)-indatraline. The research outlined above has had an important impact on the direction of our research program. With the encouraging results we have obtained, we are now solely focused on the development of green methodology and its application in the context of total synthesis. We are committed to demonstrating that it is both practical and efficient to employ eco-friendly procedures in the context of complex molecule synthesis. The undergraduate students who were involved in these projects have had their first laboratory research experience, and they have gained both practical training in synthesis and an appreciation of the importance of the role of crafting environmentally benign methods in organic chemistry. One of the students is now considering a career in chemistry and is currently inquiring about graduate level research