
ACS PRF | ACS
All e-Annual Reports

42027-AC4
Designed Hairpin Peptides with Alpha-Tetrasubstituted Amino Acids
The aims of this project were to determine the effectiveness of α-tetrasubstituted amino acids (ααAAs) as design elements in both the strand and turn portions of β-hairpins focusing on several specific aims: (1) Use of ααAAs as stabilizing elements in β-turns; (2) Effect of ααAAs on β-sheet stability in β-hairpins; and (3) Synthesis and evaluation of novel chiral ααAAs as βsheet stabilizing factors. As a result of this support three full papers and one communication have been published.
Autonomously formed β-sheet peptides are ideal models to study principles of protein folding and design. By circular dichroism and NMR spectroscopy, we have shown here thatwhen using templates with favorable cross-strand interactions, the achiral α-aminoisobutyrylglycyl (Aib-Gly) sequence acts as an effective left-handed type I' β-turn inducer with the resultant β-hairpin assuming a native-like right-handed twist (Figure 1, left). This is the same fold reported in the literature with the stereogenic DPro-Gly sequence, and found here with the stereogenic Aib-DAla sequence (Figure 1, right). The nature and stereochemistry of residues used at the i+1 and i+2 positions, while all other residues are the same, can be absolutely critical. Ithas been shown that folded structure is abolished by inversion of chirality at the i+1 position (LPro replacing DPro; conclusion based on NMR evidence) or even by removal at the i+1 position of a pro-R methyl. Additionally the Aib-Xxx turn inducing sequence circumvents the cis/trans isomerization of Zzz-Pro bonds that often complicates structural characterization.
Overall, we have shown here that when working with a template predisposed to β-hairpin formation, engineering the achiral Aib-Gly sequence in place of the commonly used, stereogenic DPro-Gly, results in equivalent folding. Thus, β-hairpins structures generated with either Aib-Gly and DPro-Gly have comparable resolution and the temperature coefficients of thetwo peptides are similar. The use of ααAAs at the i+1 position of type-I' βturns may be generalizable for use in peptide and protein design and in the development of ligands for biomolecularrecogntion, for which turn geometry and functionality are often key.
Support from PRF has also foster acollaboration with Prof. TimothyKeiderling's group at the University ofIllinois-Chicago. In this project we haveutilized the Aib-Gly hairpin peptide as a model system to study folding byinfrared (IR) spectroscopy. A series of singly and doubly 13C-(C=O) labeled peptides were prepared in our laboratoryand studied in Dr. Keiderling's group,looking specifically for unique vibroniccoupling patterns in the doubly-labeled peptides that were indicative of structure formation in the hairpin. A combination of DFT-calculations and molecular dynamics simulations assisted in the interpretation of the IR spectra. Conclusions from this work are that infrared spectra of site-specifically labeled peptides can provide structural information that iscomplementary to NMR and electronic circular dichrosim (eCD). Data strongly suggest thatthere is an ensemble of structures that are generated in the folding and unfolding of these smallpeptide systems and that a multi-step mechanism is more appropriate to understand the thermodynamic behavior of even highly folded peptides. 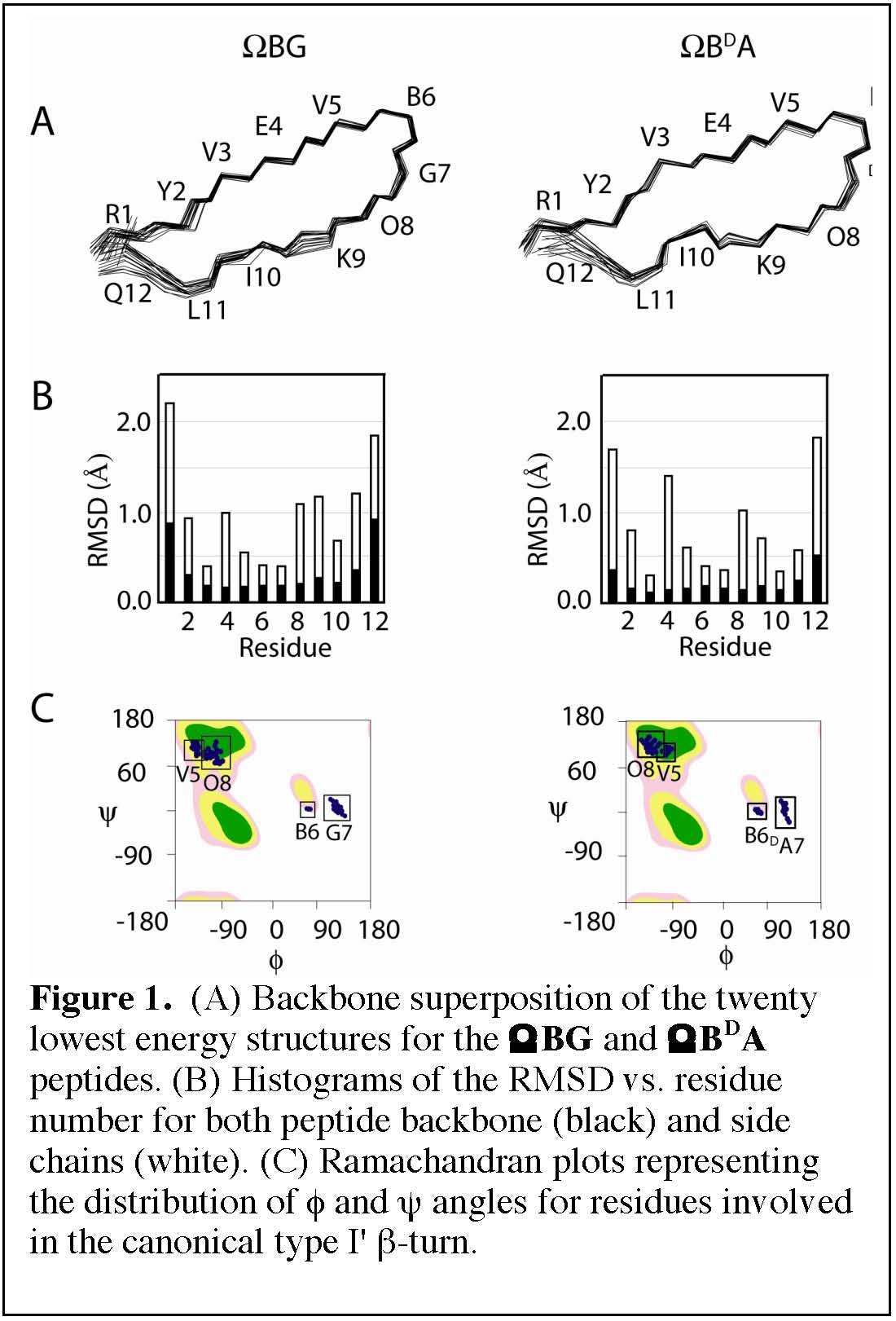
Based on results from hairpin peptides, we have investigated dipropylglycine (Dpg)sequences as potential turn inducers in a pentapeptide template. A series of Dpg and norvaline(Nva) peptides were prepared and studied by eCD, vibrational CD and NMR in water. Spectral analysis suggests that Dpg-containing peptides adopt more ordered structures relative to their Nva containing analogues. The central residues (Ala, Thr, Tyr, Val) and the charged side-chains of Glu and Lys are important to the degree of peptidefolding. Hydrophobic and branched residues (Val,Tyr) at the central position of the peptide producegreater folding. Temperature-dependent NMR analysis of Ac-Glu-Dpg-Tyr-Dpg-Lys-NH2 suggests a series of iâ†'i+3 hydrogen bonds between the N-terminal acetyl carbonyl and the Tyr3 NH, and the Glu1 carbonyl and the Dpg4 NH. The solution conformation of Ac-Glu-Dpg-Tyr-Dpg-Lys-NH2 calculated from NMR-derived constraints shows a 310 -helical structure (Figure 2), which is supported by 2D NMR, eCD and VCDspectra. Analysis of NMR-derived models of these peptides suggest that there is a strong hydrophobicinteraction of the pro-S propyl side chain of Dpg2 and the Tyr3 side-chain that is a stabilizing force of the peptide folding in water.
Unfortunately, changes in the focus of ACSPRF funding will prevent us from continuing thiswork under PRF support. One of the historical strengths of the PRF program has been its general support for strong chemical research atundergraduate and graduate institutions alike. Particularly, the support of junior faculty has been a hallmark of the ACS-PRF program. Limitations to the fields that PRF supports will greatly reduce the impact of the ACS-PRF on young faculty research programs and likely result in a reduction of the quality of research supported by ACS-PRF. Hopefully the PRF board willstrongly consider changing the current restrictions and open up the grant program to a widerportfolio of research types that served the research community so well for so many years. 