
Back to Table of Contents
44182-GB1
Functionalized Diaziridines as Potential Electrophilic Aziridinating Agents
Katherine L. Hervert, Ohio Wesleyan University
¬†The generation of easily manipulated synthetic intermediates facilitates complex molecule synthesis.( 1) The ease of formation and extensive reactivity of epoxides( 2) has underscored their synthetic utility ( 3, 4, 5). ¬†Aziridines possess a similar potential( 6) but have yet to become ubiquitous intermediates due to lack of a general aziridination method (Figure 1). ¬† Diaziridines, the nitrogen equivalent of dioxiranes, represent a promising class of aziridinating agents( 7) (Figure 2). However, their lack of applicable usage reflects the inherently lower reactivity of unsubstituted diaziridines when compared with dioxiranes. ¬†Thus a general application of diaziridines awaits a practical activation method.  The investigation of aziridinating alkenes using electrophilic diaziridines began with various synthetic attempts towards several diaziridines. ¬†Initially our efforts were focused on the isolation of diaziridine 4 (Figure 1)( 8). ¬†Our synthesis using benzaldehyde as our starting reagent did not yield product 4, suggesting our intermediate compounds had decomposed. Using the molecular modeling program CAChe, it was found that the benzaldehyde oxime had a heat of formation of 30.74 kcal/mol. ¬†This result supports our findings that 4 can not be isolated using benzaldehyde in the reaction scheme. ¬†Our data as well as other obstacles in the synthetic pathway led us to investigating synthesis of other diaziridines in the literature. ¬† Scheme 1 ¬†¬†¬†¬† 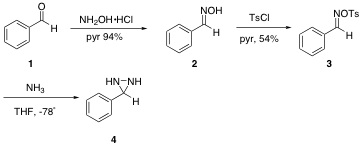 The instability of the benzaldehyde derivative led us to examine a series of diaziridines with a significantly different electronic nature. ¬†The methylphenyl-trifluoromethyl-diaziridine 9 was synthesized according to literature procedure,( ). ¬†Lithium-halogen exchange of para-bromotoluene afforded the alkyllithium of 5 which was acylated with trifluoropiperdine to give the trifluoroketone 6. ¬†Addition of hydroxylamine hydrochloride in pyridine produced oxime 7 which was tosylated to give 8. ¬†Diaziridine 9 was isolated after addition of liquid ammonia to tosyl oxime 8 at -78ňöC. ¬† Scheme 2 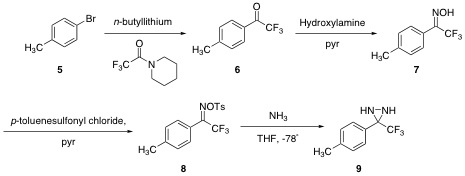 Once the diaziridine was synthesized and characterized by NMR, IR, and GCSMS, its viability as an aziridinating agent was investigated. ¬†The electron-rich trans-stilbene was the first alkene examined. ¬†Several aziridination attempts were made and the reaction progress was monitored via GCMS. ¬†Different reaction conditions were employed that varried in temperature and catalyst used (table 1). ¬†In all cases, stilbene was recovered and peaks that would correspond to the desired aziridine were not found. ¬† 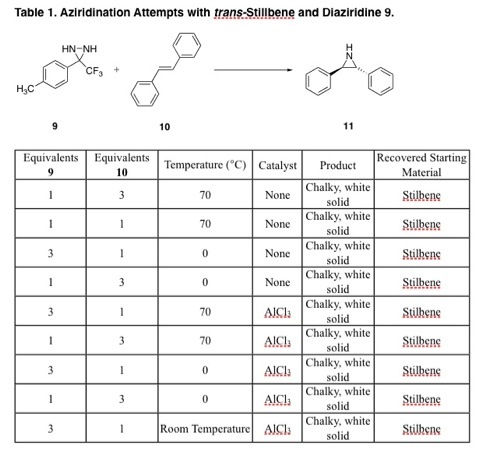 Currently, our work focuses on investigating other Lewis acids and transition metal complexes that have demonstrated aptitude for aziridination reactions. ¬†This work has also led us to examine other viable diaziridine derivatives. ¬†McCoull, W.; Davis, F. A., Synthesis, 2000, 1347. ¬†For reviews see: ¬†(a) ¬†Murray, R. W., Chem. Rev. 1989, 89, 1187. ¬†(b) ¬†Frohn, M.; Shi, Y., Synthesis, 2000, 1979. (c) Xia, Q. H.; Ge, H. Q.; Ye, C. P.; Liu, Z. M.; Su, K. X., ¬†Chem. Rev. 2005, 1603. ¬†Tanner, D. Chiral aziridines. Preparation and stereoselective transformations Angew. Chem. Int. Ed. Engl. 1994, 33, 599. ¬†Hanson, R.; Sharpless, K. B., J. Org. Chem. 1986, 51, 1922. ¬†Johnson, R. A.; Sharpless, K. B.,Catalytic Asymmetric Synthesis A. Ojima, Ed.; New York, 1993; pp159-202. ¬†¬†Atkinson, R. S., Tetrahedron 1999, 55, 1519. ¬†¬†Hori, K.; Sugihara, H.; ¬†Ito, Y. N.; ¬†Katsuki, T., Tetrahedron Letters 1999, 40, 5207. ¬†StrÝmgaard, K., Saito, D. R., Shindou, H., Ishii, S., Shimizu, T., Nakanishi, K., J. Med. Chem., 2002, 45, 4038 - 4046. 9¬†Riber, D., Venkataramana, M., Sanyal, S., Duvold, T., J. Med. Chem. 2006, 49, 1503 - 1505. ¬† Back to top
 |





