By applying the same simplification method, the percentage of bicarbonate and carbonate ions in solution can be obtained:
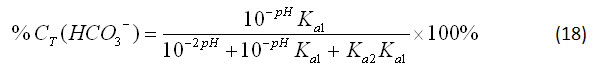
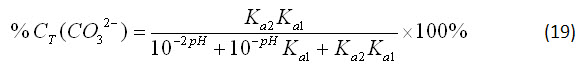
Equations 17-19 show that the speciation of carbonate in a closed system is dependent only on the pH. This is a logical result, because equations 12-14 specify that the speciation in an open system is only dependent on the atmospheric concentration of carbon dioxide and the pH, but a closed system will not interact with atmospheric carbon dioxide.
Equations 17-19 were used to plot the percentage of each carbonate species on the “Carbonates in a Closed System” graph, using the ocean pH as determined by the atmospheric CO2 slider. Just as in the graph for the open ocean, the plot for H2CO3* is labelled only as carbonic acid, although the plot depicts the percentage of total that is comprised of both carbonic acid and dissolved carbon dioxide.