Once carbon dioxide has dissolved into the ocean, carbon dioxide molecules can react with water molecules to create carbonic acid, according to equation 3:
The equilibrium constant for equation 3 is very small and the majority of dissolved carbon dioxide molecules will not react. However, aqueous carbon dioxide molecules are difficult to experimentally distinguish from aqueous carbonic acid molecules,1 so we will approximate the concentration of carbonic acid (H2CO3*) to be the sum of the concentrations of both aqueous carbon dioxide and carbonic acid.
With this approximation, equation 2 can be rewritten:
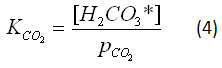
As a diprotic acid, carbonic acid can then react twice with water to form carbonate:
Equations 5 and 6, respectively, have equilibrium constants:
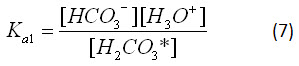
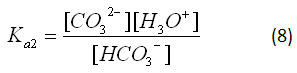
For the calculations in the applet, the values Ka1=10-5.903 and Ka2=10-9.702 were used.2 By applying Le Chatelier's principle to equation 1, it is clear that increasing the atmospheric concentration of carbon dioxide will affect the dissolution of carbon dioxide into the ocean. This will cause the equilibria given by equations 3, 5 and 6 to shift, changing the pH of the ocean, as the hydronium ion concentration changes.