Reports: AC1
47094-AC1 De Novo Synthesis of Rare/Unnatural Sugars from Achiral Materials
The O'Doherty Group Research Summary:
Overview: For the last two years the PDF has funded our group's interest in developing new methods for the de novo synthesis of natural and unnatural sugars. To these ends, we have had great success in the preparation of common and uncommon monosaccharides, and more recently, have had success in extending this methodology toward the preparation of di- and trisaccharides (vide infra).1-15
De Novo Synthesis of Monosaccharides: Our approach to produce various hexoses relies on an oxidative rearrangement of furfuryl alcohols to pyranones (Achmatowicz reaction). These furfuryl alcohols were produced via asymmetric catalysis (Sharpless oxidations/Noyori reductions). We have succeeded in developing a short route that is flexible enough for the synthesis of five of the eight possible diastereomeric hexoses and both deoxy- and 4- and 6-substituted aminosugars as either enantiomer.
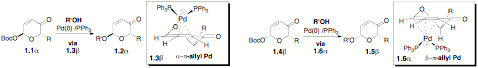
A long-term theme to this research is the development of highly stereoselective glycosylation and post glycosylation transformations that can be used in diverse complex mono-, di-, and trisaccharide settings (i.e., to be as reliable and predictable as the carbohydrate protecting group chemistry it replaces). The key to the success of this approach is the development of a mild palladium catalyzed glycosylation in combination with the discovery of a highly enantioselective approach to pyranones from acylfurans via Noyori chemistry. This approach has been expanded to a cyclitol installation reaction. This reaction has great potential for preparing various D- and L-sugars because the starting 6-t-butoxycarboxy-2H-pyran-3(6H)-ones can easily be prepared from optically pure furfuryl alcohols (either (R) or (S) form) by a two-step procedure.
Scheme 1:
De Novo Synthesis and SAR-study of Carbohydrate Natural Products: We have used the same de novo strategy to prepare several carbohydrate-based natural products. This list includes the anti-aging pheromone daumone, RSK inhibitor SL0101, anticancer/antiviral agent swainsonine, as well as, the trisaccharide portion of PI-080. The later to examples are described below (Schemes 2 and 3).
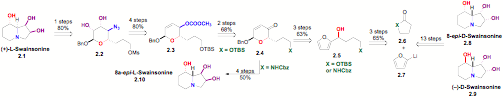
Scheme 2:
i) Synthesis of Swainsonine: We have also used this de novo asymmetric approach for the syntheses of swainsonine (2.9), its enantiomer (ent)-2.1 and several diastereomers (2.8-2.10). This highly enantio- and diastereocontrolled route illustrates the utility of the Noyori reduction, Achmatowicz reaction and palladium catalyzed glycosylation for natural product synthesis. This approach provided both enantiomers of swainsonine in 17% overall yield from furan 2.7. In addition to demonstrating the stereochemical flexibility, we desired both enantiomers, because the D-isomer is a known mannosidase inhibitor, whereas the L-isomer is a rhamnosidase inhibitor.
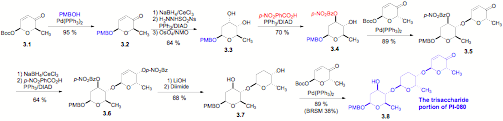
ii) PI-080 trisaccharide: We have also investigated the use of our de novo methodology for the synthesis of other biologically related trisaccharides. These efforts have culminated in the synthesis of 3.8, which is the trisaccharide portion of the aquayamycin antitumor antibiotics, PI-080. Key to the success of this approach was the discovery of a highly regioselective Mitsunobu-like inversion of the axial alcohol of a cis-1,2-diol (3.3 to 3.4). This transformation in combination with a highly diastereoselective dihydroxylation reaction becomes a nice solution to the problem of 1,2-trans-diequatorial addition to a cyclohexene.
Scheme 3:
Summary: These new approaches to carbohydrates provide a practical access to unique complex oligosaccharides that are not readily accessible by traditional carbohydrate routes.
Papers that resulted from PRF-funding period (47094-AC1)
1. "A De Novo Asymmetric Synthesis of 6-Deoxy-altro-pyranoside and its C-2, C-3 and C-2/C-3 Deoxy Congeners." M. Shan, Y. Xing and G. A. O'Doherty, J. Org. Chem. 2009, 74, 5961-5966.
2. " Structure Investigations of ent-Cladospolide D by De Novo Synthesis and Kinetic and Thermodynamic Isomerization" Y. Xing, J. H. Penn and G. A. O'Doherty, Synthesis, 2009, 2847-2854.
3. "Novo Asymmetric Syntheses of (+)-Goniothalamin, (+)-Goniothalamin oxide and 7,8-bis-epi-Goniothalamin Oxide via Asymmetric Allylation" P. Harsh and G. A. O'Doherty, Tetrahedron, 2009, 65, 5051-5055. Special issue in honor of Michael Krische's Tetrahedron's Young Investigator Award.
4. "A De Novo Asymmetric Synthesis of Cladospolide B-D: Structural Reassignment of Cladospolide D via the Synthesis of its Enantiomer" Y. Xing and G. A. O'Doherty, Org. Lett. 2009, 11, 1107–1110.
5. "A De Novo Asymmetric Approach To 8a-epi-Swainsonine" J. A. Coral, H. Guo, M. Shan and G. A. O'Doherty, Heterocycles, 2009, 79, 521-529. Special issue in memory of John Daly.
6. "De Novo Asymmetric Synthesis and Biological Evaluation of the Trisaccharide Portion of PI-080 and Vineomycin B2" X. Yu, G. A. O'Doherty, Org. Lett. 2008, 10, 4529-4532.
7. "Synthesis of Cyclitol Sugars via Pd-Catalyzed Cyclopropanol Ring Opening" M. Shan, G. A. O'Doherty, Synthesis, 2008, 19, 3171-3179. Special issue on "Cyclitol Chemistry".
8. "De Novo Asymmetric Approaches To 2-Amino-N-(Benzyloxycarbonyl)-1-(2'-Furyl)-Ethanol And 2-Amino-N-(t-Butoxycarbonyl)-1-(2'-Furyl)-Ethanol" M. H. Haukaas, M. Li, A. M. Starosotnikov, and G. A. O'Doherty, Heterocycles, 2008, 76 (2), 1549-1559. Special issue in honor of Ryoji Noyori's 70th B'day.
9. "Synthesis of Carbasugar C-1 Phosphates via Pd-Catalyzed Cyclopropanol Ring Opening" M. Shan, G. A. O'Doherty, Org. Lett. 2008, 10, 3381-3384.
10. "De Novo Asymmetric Synthesis of Anthrax Tetrasaccharide and Analogue" H. Guo, G. A. O'Doherty, J. Org. Chem. 2008, 73, 5211-5220. (Featured Article)
11. "Formal Total Synthesis of RK-397 via an Asymmetric Hydration and Iterative Allylation Strategy" H. Guo, M. S. Mortensen, G. A. O'Doherty, Org. Lett. 2008, 10, 3149-3152.
12. "De Novo Asymmetric Synthesis of the Trisaccharide Subunit of Landomycin A and E" M. Zhou, G. A. O'Doherty, Org. Lett. 2008, 10, 2283-2286.
13. "Metabolite induction of Caenorhabditis elegans dauer larvae arises via transport in the pharynx" T. J. Baiga, H. Guo, Y. Xing, G. A. O'Doherty, A. Parrish, A. Dillin, M. B. Austin, J. P. Noel, James J. La Clair, ACS Chem. Biol. 2008, 3, 294-304.
14. "De Novo Asymmetric Synthesis of 8a-epi-Swainsonine" J. N. Abrams, R. S. Babu, H. Guo, D. Le, J. Le, J. M. Osbourn and G. A. O'Doherty, J. Org. Chem. 2008, 73, 1935-1940.
15. "De Novo Asymmetric Syntheses of D-, L- and 8-epi-Swainsonine" H. Guo and G. A. O'Doherty, Tetrahedron, 2008, 64, 304-313.