Reports: G4
48202-G4 Substituent Effects on the Photochemistry of 1,4-Disubstituted Tetrazolethiones
Our proposal outlined goals to investigate the substituent effects on the photochemistry of tetrazolethiones with the goals of applying these ring systems for synthesis of photoreactive polymers. We have made significant progress on this project as summarized below:
A.1 Syntheses of tetrazolethiones 4ab
1,3-dipolar cycloaddition of phenyl isocyanates 1a-b with trimethylsilyl azide yielded the 1-phenyl-5H-tetrazoles 2a-b. The latter were subjected to methylation to obtain 1,4-disubstituted tetrazole-5-ones 3a-b that upon treatment with Lawessons reagent yielded the 1-methyl-4-phenyl-1H-tetrazol-5(4H)-thione 4a and 1-methyl-4-(3-methoxyphenyl)-1H-tetrazol-5(4H)-thione 4b. All compounds were spectroscopically fully characterized.
Scheme 1. Synthesis of tetrazolethiones 4a - b.
A.2 Absorption and fluorescence characteristics of 4ab
The absorption spectra of 4a-b were recorded in cyclohexane, THF and acetonitrile. Four bands were observed that showed minimal dependence on the polarity of the solvent. Time-dependent density functional calculations revealed that all bands result from p→p* excitations with some degree of intramolecular charge transfer within the molecules. Our results further suggested that UV excitations of 4ab are not dominated by transitions from one molecular orbital to the other, but several excitations occurring between different molecular orbitals of similar energy contribute to the formation of an absorption band. TDDFT studies also demonstrated that the transitions to the second or higher excited states are characterized by oscillator strengths that are orders of magnitude higher than the oscillator strength of the first excited state (S1). This supports that the higher excited states (S2, S4, S6 etc.) may play a crucial role in the photochemistry of 4a-b. The fluorescence spectra of 4ab were obtained in acetonitrile at excitation wavelength of 254 nm. Both compounds showed a broad absorption near 315 nm.
A.3 Products of photodecomposition of 4ab
Photolyses of 4a-b were carried out in a Rayonett reactor at 254 and 300 nm in solvents of varying polarity (e.g. benzene-d6, acetonitrile-d4 and methanol-d4). The reaction mixture was analyzed by NMR spectroscopy. The primary photoproduct of photolyses was the respective carbodiimide 5a-b formed by the loss of dinitrogen and sulfur. No solvent dependency on product yields or rate of photodecomposition was observed that supports a non-ionic pathway for photodecomposition of tetrazolethiones.
Scheme 2. Formation of carbodiimide during the irradiation of 4a-b.
A.4 Excited states in the photochemistry of tetrazolethiones
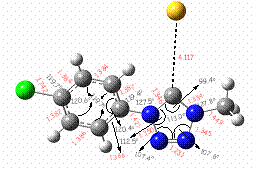
We have performed geometry optimization on a related compound, 1-methyl-4-(4-chlorophenyl)-1H-tetrazol-5(4H)-thione at RHF/6-31G+**. The excited states were determined at CIS level of theory using RHF/6-31G+** ground state geometries. Our initial computational studies predict a substantial lengthening of the C-S bond in the excited state (4.117 Å) which indicates that the photodecomposition of tetrazolethiones may proceed via the formation of a heterocyclic carbene (Figure 1). The latter could be envisioned to form carbodiimide, the major photoproduct of tetrazolethione photochemistry (Section A.3), through the loss of dinitrogen.
Figure SEQ Figure \* ARABIC 1. Excited State (root = 8) for 1-methyl-4-(4-chlorophenyl)-1H-tetrazol-5(4H)-thione optimized at CIS/6-31+G*
A.5 Mechanism of photodecomposition of 4ab
A.5.1 Investigating the intermediacy of a carbene. In order to study the intermediacy of a heterocyclic carbene in the photodecomposition of 4a-b as suggested by our ab initio studies (Section A.4), we carried out the photolysis of 4b in cyclohexene using medium pressure mercury lamp. The analyses of the crude reaction mixture using ESI-MS/MS indicated the presence 7 that corresponded to the trapped carbene 6 (Scheme 3). All the attempts to isolate 7 from our reaction mixtures have proved to be futile.
Scheme 3. Photolysis of 4b in the presence of cyclohexene.
A.5.2 Investigating the intermediacy of a biradical. Studies by others on tetrazolones have revealed that their photodecomposition occur via the formation of a triplet biradical (Friza 2006 and 2008). Therefore, we investigated the possibility of existence of a biradical in the mechanism of photodecomposition of 4a-b. We carried out the photolysis of 4a-b in the presence 1,4-cyclohexadiene (300nm, methanol-d4). Our results showed the formation of thioureas 9a-b as the major product via the biradicals 8a-b. Only trace amounts of the carbodiimides 5a-b were formed.
Scheme 4. Photolysis of 4a-b in the presence of 1,4-cyclohexadiene.
In order to gain further insights into the mechanism, we have investigated the photochemistry in triplet sensitizers of varying energies (e.g. benzophenone, acetophenone and acetone) at 300 nm in methanol-d4. The analyses of the reaction mixture by NMR spectroscopy revealed that no carbodiimides 5a-b formed and therefore, no sensitization was observed. In order to rule out the possibility of high lying triplet state or a short lived triplet state, we also performed the photolyses in the presence of triplet quenchers (e.g. 1,3-pentadiene and 1,3-cyclohexadiene) in methanol as the solvent. To our surprise, the analyses of the reaction mixture indicated the formation of 9a-b as the major products with the formation of 5a-b as the minor products. The decomposition rates of 4a-b were found to be higher in the presence of quenchers. This may be because of their efficient ability to act as H-donors to the putative biradical 8a-b.
A.6 Summary
Our results strongly suggest the intermediacy of a biradical during the photodecomposition of 4a-b. The mechanism of photodecomposition of tetrazolethiones may also involve the formation of a heterocyclic carbene, however, it is certainly not the major pathway. Our ongoing efforts in this area are focused on employing Laser Flash Photolysis to gain further insights into the mechanism. We are also extending our studies to a series of other tetrazolethiones to create a comprehensive knowledge base of substituent effects on the photophysical and photochemical properties tetrazolethiones.
A.7 Impact of this award We are just beginning to understand the mechanism of photodecomposition of tetrazolethiones. Our goal is to employ tetrazolethiones for the synthesis of photoreactive polymers with a variety of applications. The results being generated from our ongoing efforts will serve as preliminary data for a full proposal to the National Science Foundation. We are preparing to submit a manuscript on the results reported in this report which will allow my research group visibility in the scientific community. All of this would have otherwise been not possible without the support from ACS-PRF.