Reports: AC3
47899-AC3 Rational Syntheses of Metallocarbohedrynes (Met-cars)
Introduction
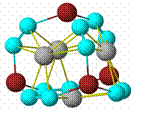
The original proposal was to develop rational syntheses of metallocarbohedrynes (met-cars) such as Ti8C12 (pictured). This class of compounds had heretofore been known only in the gas phase at high temperatures, and syntheses in the condensed phase are clearly desirable because of the known (experimentally) and anticipated (from computational studies) properties of this class of compounds. Thus the most studied met-car, Ti8C12, has an ionization potential which lies between those of sodium and potassium and a HOMO-LUMO gap near zero, properties which are probably unique for molecular species and which suggest metallic properties and extremely interesting chemistry in the condensed phase. The original proposal outlined other features which added to our interest in this class of molecules.
Comments on Procedures
Space limitations prevent a broad overview of all the experiments carried out to date. The experiments described below are therefore only representative.
Taking the C2 units as acetylidic (C22-) in nature, then the average formal oxidation state of the titanium atoms in Ti8C12 is +1.5 and we proposed a general synthetic approach which would involve combining readily available sources of C22- with readily available soluble titanium precursors in the +3 and +4 oxidation states in the presence of reducing agents capable of reducing the Ti(III) and Ti(IV) precursors. Attempts would also be made to synthesize met-cars containing other metals and mixed metal species.
Titanium Sources
Initially, TiCl4 was intended to be used as a source of titanium, but this compound is a very readily hydrolyzed liquid which fumes in air. TiCl4 complexes with acetonitrile, tetrahydrofuran and tetramethylethylenediamine are more stable crystalline solids which were found to be much more convenient to work with. Analogous titanium(II) and -(III) complexes have also been synthesized and employed as titanium sources.
Carbon Sources
Acetylene was synthesized initially via hydrolysis of calcium carbide, but the latter is a very hard solid which was difficult to obtain as a high surface area powder and most of our experiments with acetylene have utilized pressurized cylinders. A problem here is that the acetylene is pressurized in acetone solution and purification of the acetylene gas stream was necessary. This was achieved by passage of the acetylene/acetone gas stream through deionized water, concentrated sulphuric acid and activated 3A molecular sieves in that order. Another source utilized was sodium acetylide, NaC2H, which is commercially available.
Reducing Agents
We have experimented at various times with sodium amalgam, sodium and lithium metals and sodium naphthalenide as reducing agents. All have advantages and disadvantages, but all do effect reduction of Ti(III) and Ti(IV) compounds as evidenced by color changes.
Analytical Methods for Sample Analyses
In view of the low ionization potential and near zero HOMO-LUMO gap of Ti8C12, one might well expect this material to be obtained in condensed phase as a black, insoluble, metallic solid. As such we would have recourse to elemental analyses, IR spectroscopy and x-ray photoelectron spectroscopy (XPS). The vibrational spectrum of Ti8C12 has been reported (nC=C identified) and comparisons could be made, while XPS would be useful as the two types (inner and outer) of titanium atoms should have different binding energies and therefore be distinguishable.
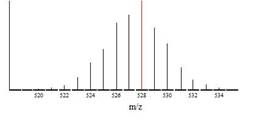
Electrospray ionization (ESI) mass spectrometry was also expected to be useful because of the very low ionization potentials of met-cars (see above). No matter how much effort we might make to achieve anaerobic and anhydrous reaction conditions, slight oxidation could be anticipated and low concentrations of met-cars cations might well be detectable in reaction solutions. The very distinctive isotope pattern for the cluster species Ti8C12+ (see figure) would be obvious.
Representative Attempts at the Synthesis of Ti8C12 (studies of all of these continue)
1. Acetylene was bubbled through a THF solution of TiCl4 stirring over sodium amalgam and a black tar-like substance was obtained. ESI MS of the supernatant exhibited many peaks, of which those centered at 646 m/z and 718 m/z appear interesting as they exhibited isotope patterns consistent with their containing several titanium atoms.
2. TiCl2(THF)2 was reacted with acetylene in THF in the absence of a reducing agent; this pre-reduced precursor was used because of the possibility of disproportionation. The reaction resulted in the formation of a black precipitate which exhibited a metallic luster when viewed under a microscope. As noted above, these observations (color, luster) are consistent with the expected physical properties of bulk Ti8C12, although the appearance did not change when the sample was exposed to air for many hours. Diffuse reflectance IR spectroscopy (DRIFTS) spectra exhibited a strong peak at 1395 cm-1, possibly attributable to Ti8C12, but there were also peaks at 3012 cm-1 and 1013 cm-1 which are similar to published CH and CC stretching bands of polyacetylene. While the latter is in retrospect a possible product, the X-ray powder diffraction pattern and the apparent oxidative stability of the material are inconsistent with published data for polyacetylene and research on this and similar products continue. For instance, a number of experiments involving acetylene have yielded black, insoluble materials with metallic lusters (silver or gold colored) when viewed under a microscope. However, while DRIFTS and powder diffraction experiments suggest possible similarities to polyacetylene, the materials are not all identical and they do not conform in detail to descriptions of polyacetylene in the literature. In view of the similarities in their properties with those anticipated for Ti8C12, we continue to work with them.
3. Reduction of TiCl4(THF)2 with sodium amalgam in the presence of sodium acetylide yielded a grey material. An ESI MS spectrum of a solution in MeCN exhibited many peaks, including a cluster centered at 852-868 amu and which has an isotopic expected for [Ti8C12(CH3CN)8]+. We have had difficulties repeating this experiment and so there are details which we have not yet worked out. Thus we continue to work with this system.
4. Reduction of TiCl4(THF)2 with sodium naphthalenide in THF in the presence of sodium acetylide yielded a black material for which an ESI MS spectrum in methanol exhibited several multiplets with the desired isotopic distributions. Work on this system also continues.