Reports: AC2
48445-AC2 Microbial Biogeochemistry of Isoprenoid Hydrocarbon Cycling
soprenoid compounds, polymers isoprene, are biologically produced molecules ubiquitous to the three domains of life. Enhanced stability, particularly in anoxic settings, is conferred by methyl branching of the alkyl chains. Long term preservation makes isprenoids useful geologic biomarkers with both taxonomic and paleoenvironmental specificity. For example, glycerol diether glycerol tetraethers (GDGT's) are indicative of archaea and show a relation to temperature, salinity and nutrient concentrations [1, 2]. Evidence exists for a microbial role in the anaerobic breakdown of isoprenoids through simultaneous changes in hydrocarbon profiles, gas evolution and electron acceptor concentrations. However, no information on the phylogeny and biochemistry of this process has been reported [3, 4].
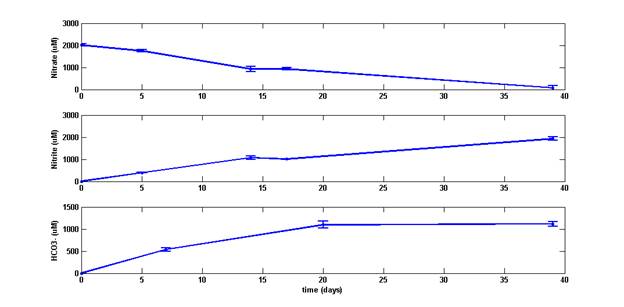
Here we describe pristane (2,6,10,14-tetramethylpentadecane) biodegradation and the accompanying loss of nitrate in an activated sludge enrichment. Nitrate consumption and nitrite prodution are monitored using ion chromatography. Bicarbonate production is monitored using a GC-TCD. Over forty days, enrichments consumed 2 mM nitrate and produced 1.1 mM bicarbonate and 1.9 mM nitrite. Pristane (98.6% ChemService) was determined to contain trace amounts of phytane and squalane by GC-MS. After forty days of growth, changes in the ratios of pristane/phytane (Pr/Ph 56.5), pristane/squalane (Pr/Sq 11.7) and phytane/squalane (Ph/Sq 0.21) indicate preferential loss of pristane to both phytane and squalane in active cultures as compared to controls (Pr/Ph 67.0, Pr/Sq 14.9 and Ph/Sq 0.22).
Figure 1: Nitrate consumption (top) acompanied by nitrite (middle) and bicarbonate (bottom) production in denitrifying, pristane degrading enrichments.
We followed the evolution of the enrichment community using 16S rDNA clone libraries and fluorescence in situ hybridization (FISH). We obtained the original innocula from waste water treatment activated sludge (University Park, PA). Enrichments of this inocula showing growth on pristane were propogated through sequential transfers. A comparison of clone libraries from the second and fourth transfer shows a decrease in bacterial diversity and a greater percentage of clones related to denitrifying β-proteobacteria and a denitrifying, hexadecane degrading γ-proteobacterial isolate. Consistent with changes observed in clone libraries, FISH experiments show an increase in cells hybridizing with probes specific for β-proteobacteria (BET42a) and γ-proteobacteria (GAM42a) with successive transfers.
The current enrichment degrades pristane as well as archaeal diether lipids, but not archaeal tetraethers lipids. Experiments in progress are designed to establish reaction stoichiometries and to monitor intermediates that may yield insight into the degradation pathway. These data will have implications for the fate of biomarkers in modern anoxic environments and in geologic reservoirs.
1. Wuchter, C., et al., Archaeal tetraether membrane lipid fluxes in the northeastern Pacific and the Arabian Sea: implications for TEX86 paleothermometry. Paleoceanography, 2006. 21.
2. Turich, C., et al., Lipids of marine Archaea: Patterns and provenance in the water-column and sediments. Geochimica et Cosmochimica Acta, 2007. 71(13): p. 3272-3291.
3. Bregnard, T.P., et al., Anaerobic Degradation of Pristane in Nitrate-Reducing Microcosms and Enrichment Cultures. Appl. Environ. Microbiol., 1997. 63(5): p. 2077-2081.
4. Grossi, V., et al., Anaerobic biodegradation of pristane by a marine sedimentary bacterial and/or archaeal community. Organic Geochemistry, 2000. 31(7-8): p. 769.