Reports: B1
45277-B1 Investigation of the Butyllithium-Induced Sigmatropic Rearrangement: Intramolecular Cyclization Reaction of Allyl-Dichlorovinyl Ethers
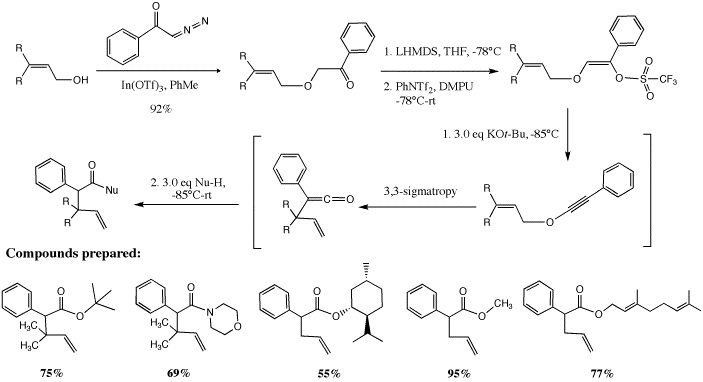
Our research for the grant period has focused on the formation of alkynyl ethers and the sigmatropic rearrangement of allyl and benzyl alkynyl ethers. We have previously developed an improved procedure for the formation of allyl alkynyl ethers, which undergo rapid low-temperature sigmatropic rearrangement to furnish gamma, delta-unsaturated esters. The reaction of allylic alcohols and alpha-diazoketones in the presence of indium triflate as catalyst rapidly furnishes alpha-alkoxyketones. Enol triflates derived from these substrates undergo a facile E2 elimination at –78°C when treated with potassium tert-butoxide to furnish allyl alkynyl ethers, which immediately undergo sigmatropic rearrangement and trapping with an alcohol to furnish the unsaturated ester products.
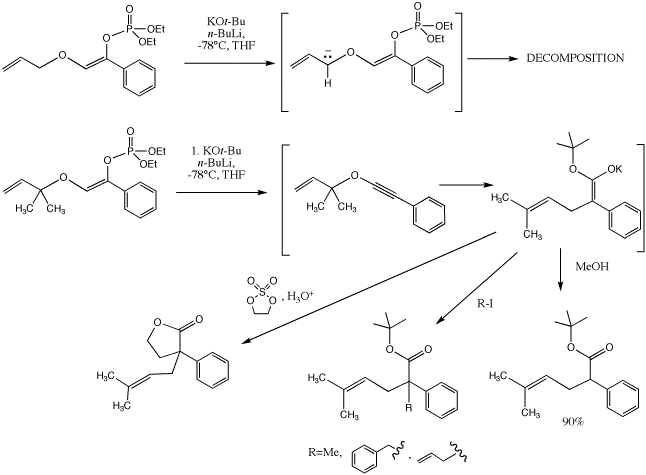
We have also shown that enol phosphates derived from alpha-alkoxyketones produce alkynyl ethers when exposed to Schlosser's base (n-BuLi/KOtBu) at low temperatures (-78°C). The enol phosphates have significantly greater stability than the enol triflates, and we hypothesized that they may also be able to undergo [3,3]-sigmatropic rearrangement to form ketene intermediates that may be trapped with carbon nucleophiles in an inter- or intramolecular fashion. Indeed, we discovered that treatment of allylic enol phosphates with n-BuLi in the presence of KOtBu led to rapid production of the rearranged gamma, delta -unsaturated tert-butyl ester in 90% yield. Subsequently we realized that it was also possible to trap the ester enolate intermediate with electrophiles (such as alkyl, allyl, and benzyl halides) to furnish alpha-substituted tert-butyl esters. Gamma-Butyrolactones may be prepared by addition of a cyclic sulfate to the ester enolate.
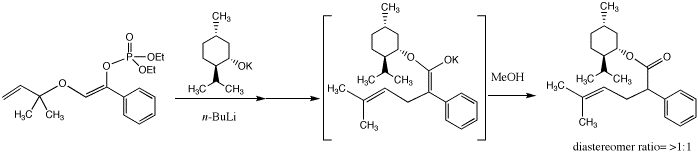
Finally, we are currently in the process of investigating the use of chiral alcohols in the rearrangement process, which would give rise to products with diastereomeric excess. Thus, we have found that employing potassium menthoxide instead of potassium tert-butoxide in the deprotonation of allyl enolphophates leads to the corresponding menthyl ester product with ~3:1 diastereomeric excess. Studies investigating the trapping of the intermediate chiral ester enolates with a variety of electrophiles are underway, and we are hopeful of obtaining products containing quaternary stereogenic centers in high diastereomeric excess.
With the encouraging results we have obtained, we are now focused on the application of this methodology in the context of total synthesis.
The undergraduate students who were involved in these projects have had their first laboratory research experience, and they have gained practical training in synthesis. One of the students is now considering a career in chemistry and is currently inquiring about graduate level research