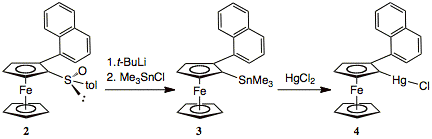Reports: AC3
45648-AC3 Lewis Acid Chemistry at the Edge of Ferrocene
Bidentate Lewis acids have attracted much recent interest due to their superior performance in diverse areas ranging from Lewis acid catalysis of organic and organometallic reactions, activation of catalysts in olefin polymerization, selective and efficient molecular recognition of anions and neutral nucleophiles, to new electronically interesting materials. The purpose of the research under this grant was to explore the chemistry of ferrocenes with two adjacent Lewis acid centers attached to one of the cyclopentadienyl rings and to develop the related planar chiral analogs. While the more easily accessible 1,1'-dimetalated ferrocenes have been widely studied in the past, the chemistry of the related 1,2-difunctionalized ferrocenes is virtually unexplored. The latter are of particular interest since they represent three-dimensional, redox-active analogs of the well-established and highly useful bidentate Lewis acids with phenylene and naphthalene backbones.
During the current grant period we have focused on the following two tasks.
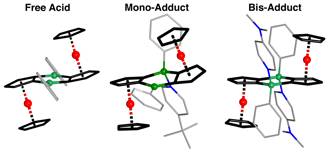
(A) We have studied the binding behavior of diboradiferrocene (1), in which two adjacent Lewis acidic organoboron moieties lead to a doubly-bridged diferrocene system.
The 1:1 and 1:2 adducts with different pyridine derivatives (4-t-butylpyridine (tBupy) and 4-dimethylaminopyridine (DMAP)), were obtained by low temperature crystallization or slow solvent evaporation techniques. Single crystal X-ray diffraction data were collected for the 1:1 adduct with DMAP and for both 1:2 adducts, which revealed marked structural changes as a result of pyridine binding (Figure 1).
In solution, the 1:2 adducts were found to readily undergo dissociation with formation of the 1:1 adducts as the dominant species at room temperature. Variable temperature NMR and UV-visible titration studies demonstrated that the first binding process is more than five orders of magnitude more favorable than the second binding process.
Figure 1. Molecular structures of the free acid 1, as well as the 1:1 and 2:1 pyridine adducts.
(B) We have also developed a new approach to planar chiral ferrocenylboranes and isolated a highly Lewis acidic perfluorinated ferrocenylborane derivative (5) in enantiomerically pure form. We applied a directed ortho-lithiation approach of 2, which was followed by metathesis with organotin and mercury halides. All these reactions proceded with excellent yields (Scheme 1).
Scheme 1. Synthesis of chiral metalated ferrocene species.
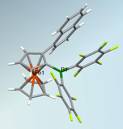
The utility of these organometallic species as precursors to ferrocenylboranes was next examined. We found the ferrocenylmercury derivative 4 to give by far the best selectivity and highest yields. The highly Lewis acidic chiral organoborane 1-naphthyl-2-bis(pentafluorophenyl)borane (5) was obtained in high yield by treatment of the respective chloroborane with the mild reagent CuC6F5. Studies on applications of this unusual Lewis acid in catalysis are currently under way.
Figure 2. Single crystal X-ray structure of 1-naphthyl-2-bis(pentafluorophenyl)borylferrocene (5)