Reports: GB3
47420-GB3 Bio-Inspired Two-Site Bimetallic Hydrocarbon Activation Catalysts
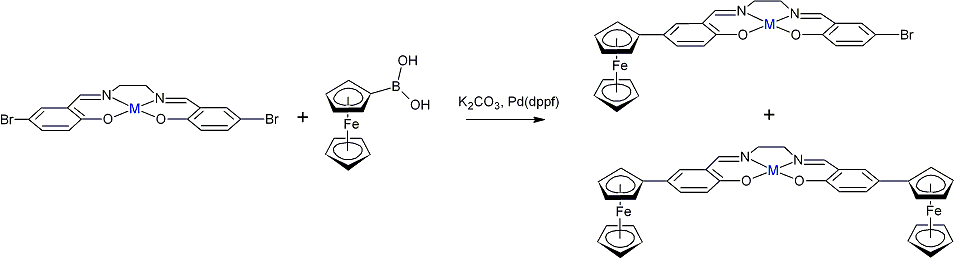
Research Progress. This overall goal of this research is the preparation of bimetallic oxidation catalysts that mimic enzymes by employing tethered redox cofactors to accomplish multi-electron oxidations. The original proposal called for the preparation of redox-active metallocene derivatives of metal salen and porphyrin complexes and the characterization of their reactivity towards molecular oxygen. Work during the present grant period has largely focused on the former goal. In particular, I have worked with three undergraduate researchers to explore two parallel strategies for preparing such complexes: (a) covalent attachment of the ferrocene group directly to a salen ligand and (b) the preparation of ferrocene-containing axial ligands. Early efforts focused on Suzuki coupling between ferroceneboronic acid and M(5,5'-dibromosalen) (M = H2, CoII), as shown below:
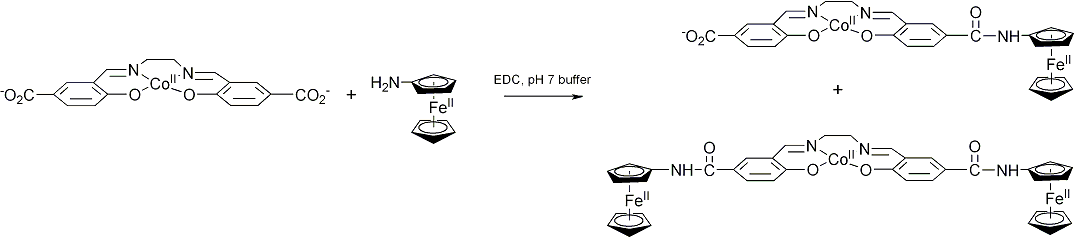
These reactions produced mixtures of poorly-soluble mono- and disubstituted products which proved difficult to separate chromatographically using either silica gel or organic resins. The brown product mixture slowly reacts with atmospheric oxygen to afford an intractable green solid. Although we are not certain, it is likely that the color change is due to oxidation of the ferrocene group to ferrocinium. In order to prepare more soluble ferrocene derivatives, we investigated derivatives of 5,5'-dicarboxysalen. EDC coupling between CoII(5,5'dicarboxysalen) and aminoferrocene produced a mixture of air-sensitive products which are somewhat soluble in water, alcohols, and acetonitrile and which can be separated using chromatography. Currently, we are engaged in efforts to structurally characterize these products and their oxygen reactivity.
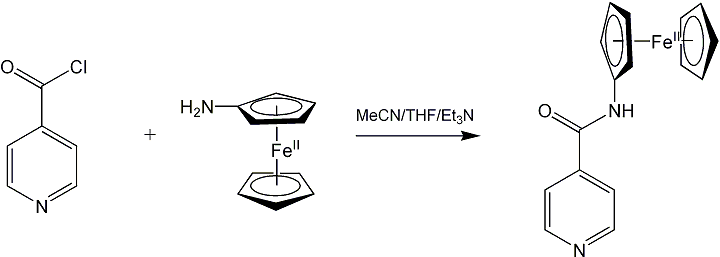
We also explored the preparation of ferrocene-containing axial ligands that could be used to alter the oxygen reactivity of conventional salen and porphyrin complexes. So far, we have successfully prepared a ferrocene-containing pyridine derivative shown below:
We investigated the interactions between CoII(5,5'-Me2salen) and this ligand using a combination of electrochemistry and spectroscopy. Our results so far indicate that they do not interact in donor solvents but form an oxygen-sensitive adduct in dichloromethane. Furthermore, the adduct reacts with molecular oxygen to give a green solid that is soluble in methanol but insoluble in water, dichloromethane, and nonpolar solvents. We are currently engaged in efforts to characterize this adduct and the product of its reaction with molecular oxygen using X-ray crystallography and cyclic voltammetry.
In addition to our work described above, we also attempted to investigate whether 1 alters the reactivity of FeII-picket fence porphyrin complexes. However, these efforts have been largely abandoned after finding that undergraduate researchers had difficulty making adequate progress in preparing these complexes within the context of a 10 week summer research program.
Career impact. Since Westmont College chemistry department has been generous in providing funds for supplies and equipment, the majority of this grant provided stipend support that enabled me to devote two summers to working with undergraduate researchers. The work we accomplished during these summers laid the foundation for my research program involving undergraduates. We successfully developed techniques for preparing and handling redox-active metallocene derivatives of metal salen complexes. Furthermore, the preliminary results we obtained indicate that the complexes do exhibit reactivity toward oxygen that differs from that of mixtures of CoIIsalen and ferrocene. Once we have finished fully characterizing the structures and oxygen reactivity of these complexes, I anticipate that we will be able to submit one to two papers detailing our results.
In addition to my own professional development, the grant provided three different undergraduate students with research experience during the summers of 2008 and 2009. One of these, Cherol Tomer, plans on becoming a High School chemistry teacher and is currently enrolled in the teaching credential program at San Jose state University. The remaining students, William Farnsworth and Eric McDonald, will be completing their studies this year and plan on applying to medical schools next year. Of these, William is continuing his work on this project during the current school year. I anticipate using the remaining grant funds to support two additional research students during the summer of 2010 and, if granted a no-cost extension, to support two students during the summer of 2011. In fact, this project has already attracted an additional undergraduate researcher, Bryan Brautigham, who is planning on continuing these projects in the summer of 2010 and throughout the 2010-2011 academic year.