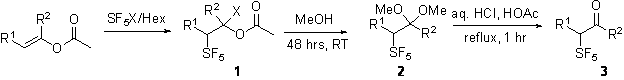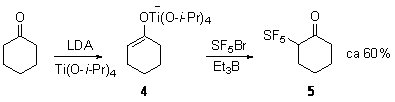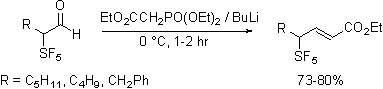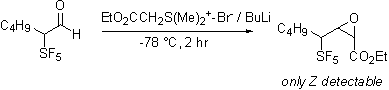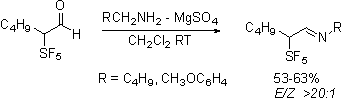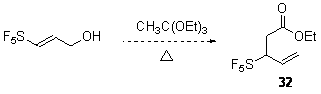Reports: AC4
46219-AC4 The Preparation of Optically Active Pentafluorosulfanylated Building Blocks: Selectivity and Reactivity in Synthesis
I. Introduction. The pentafluorosulfanyl (SF5) group is one of only a very few truly new functional groups to be introduced to the armentarium of the synthetic organic chemist in the last 100 years. The pseudooctahedral symmetry of the SF5 group, presenting a square pyramid of electron density, as defined by the fluorine ligands, is not otherwise known to the medicinal or pharmaceutical chemist. Although this functional group has very recently found applications as an aromatic substituent in agrochemicals, pharmaceuticals and liquid crystals, in aliphatic chemistry, pentafluorosulfanylated materials are only very rarely encountered with applications largely limited to polymer or oligomer preparations. The profoundly electron withdrawing nature of the SF5 group when combined with the highly polarizable carbon-sulfur bond may directly influence reactivity in manners different from those associated with the trifluoromethyl group. The hypotheses to be tested in the proposed research are; firstly that the electronic influence of the pentafluorosulfanyl unit can be used to direct the stereochemistry of reactions of a pentafluorosulfanylated molecule and secondly that pentafluorosulfanylated building blocks can be converted to carbohydrates, amino acids or other biologically active molecules.
II. . Preparation and Purification of Starting Aldehydes and Ketones. Over the last two years we have prepared building blocks for the study of the electronic influence of the SF5 group. The goal was to reproducibly prepare α-pentafluorosulfanyl aldehydes and ketones. We have learned that the pentafluorosulfanyl bromide addition to an enolate or enol acetate is both effective and efficient. (See Table 1)
Use of enol acetates to first form 1 and finally hydrolysis directly to 3 or thru the intermediate 2 is scaleable.
Table 1. Synopsis of Preparations.
|
Entry |
R1 |
R2 |
SF5X |
Yield |
Yield |
Yield |
|
a |
C5H11 |
H |
SF5Br |
43% |
71% |
82% |
|
b |
C3H7 |
H |
SF5Br |
74% |
62% |
24% |
|
c |
C4H9 |
H |
SF5Br |
88% |
76% |
81% |
|
d |
PhCH2 |
H |
SF5Br |
- |
76% |
X |
|
e |
PhCH2CH2 |
H |
SF5Br |
27% |
91% |
49% |
|
f |
H |
H |
SF5Br |
- |
97% |
- |
|
g |
H |
Ph |
SF5Cl |
- |
- |
44% |
|
h |
H |
C6H13 |
SF5Cl |
66% |
- |
- |
|
i |
C2H5 |
C3H7 |
SF5Cl |
15% |
- |
- |
|
j |
C2H5 |
C3H7 |
SF5Br |
50% |
- |
- |
|
k |
H |
CH3 |
SF5Cl |
92% |
- |
- |
More direct access to SF5-containing enolates is possible via the titanium ate enolate 4 that will react directly with SF5Br in hexane to form 5.
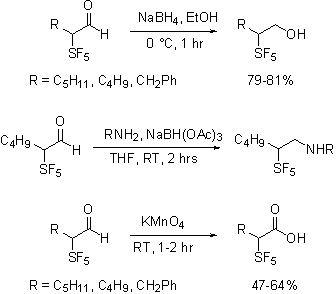
IV. Summary of Findings. Reactions of Aldehydes. The SF5-containing aldehydes can be subjected to a variety of transformations. To establish the scope and generality of reactions of SF5-containing aldehydes and the stability of the products, those aldehydes have been subjected to a variety of routine transformations, such as sodium borohydride reduction, reductive amination, and permanganate oxidation.
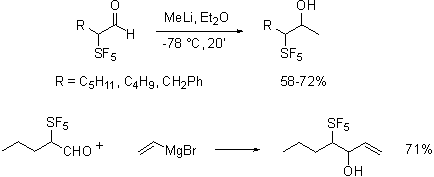
As shown the reactions were uneventful, in contrast to published findings we have found that Grignard and organolithium additions to the aldehyde are especially clean reactions.
Yamazaki has elegantly rationalized selectivity of 2-CF3-propanal by Felkin-Anh control. (See below)
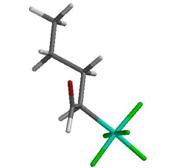
Preferred conformation of SF5-pentanal (6-31 G** B3LYP) is associated with a 35% reduction in dipole moment. Note the synperiplanar alignment of the carbonyl group with with the alkyl chain.
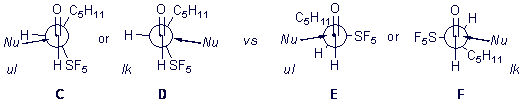
Since addition of vinyl magnesium bromide to the aldehyde forms a single diastereomer unlike additions to trifluoromethylated aldehydes and the relative diastereochemistry of the product was determined to ul by NMR methods. This is strongly suggestive of dipolar control of addition rather than orbital interaction control..
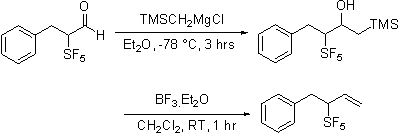
In contrast, olefination reactions are sensitive to the nature of the reagent employed. Horner-Emmons reagents add to give easily purified and characterized materials. In contrast Wittig reagents, phosphonate ylids, diphenylsulfonium cyclopropylide and trimethylsulfoxonium ylides do not react cleanly.
Dimethylsulfonium carboethoxymethylide does react selectivity forming only the Z expoxide. With the observation that the apparently less basic ylides react selectively it was found that Peterson olefination via the intermediacy of silyl alcohol also proceeds cleanly. Apparently the increased acidity of the proton alpha to the SF5 group is responsible for the complexity of the reaction products.
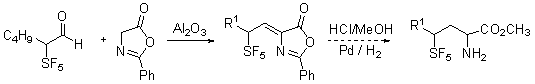
In spite of the steric congestion associated with reaction of the SF5-containing aldehydes we have also found that formation of an Erlenmeyer azlactone, the intermediate to homologative amino acid synthesis, is possible.
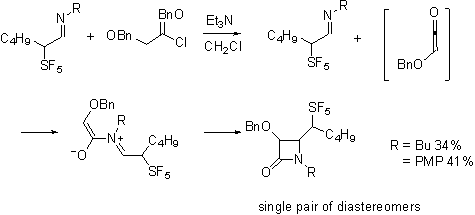
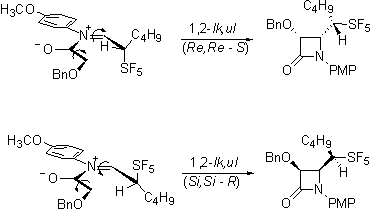
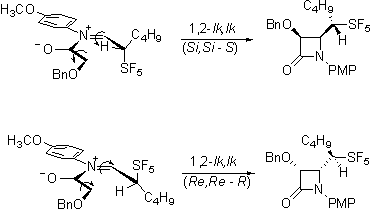
The most exciting evidence of stereoselectivity influenced by the pentafluorosulfanyl group is in the Staudinger reaction of the aldehydes. Not unexpectedly, the required imines are formed stereoselectively.
Interestingly the ketene-imine cycloaddition reaction, occurs selectively forming only a single diastereomeric pair from the racemic imines.
Our current efforts are directed toward determining whether this product is the lk,ul diastereomeric pair that would be consistent with the Felkin-Anh model and with the work of Yamazaki on trifluoromethylated imines.
Or is rather the lk,lk pair that would be consistent with a Cieplak description of the reaction.
It is important to note that the exploration of the influence of the pentafluorosulfanyl group on reactivity and selectivity is slowed as we move further from the initial SF5Br reagent. It is the is a necessity to prepare larger and larger amounts of the pentafluorosulfanyl containing building blocks in order to explore the reaction conditions and stability of the products that has led us to focus on those reactions that form products most cleanly to facilitate isolation and characterization.
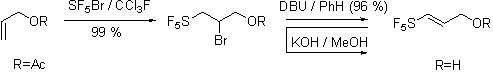
However our work on [3,3]-sigmatropic rearrangements is currently in progress. We have focused our preliminary experiments on the Johnson ortho-ester Claisen rearrangement of pentafluorosulfanyl containing allyl alcohol prepared as shown.
Our preliminary investigations of the Johnson orthoester Claisen procedure are consistent with the Bronsted acid stability of aliphatic pentafluorosulfanyl group and indicate that the reaction proceeds albeit slowly.