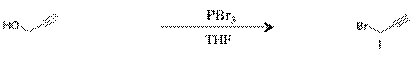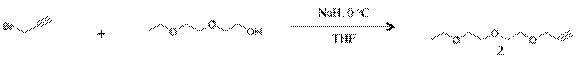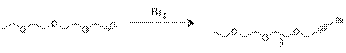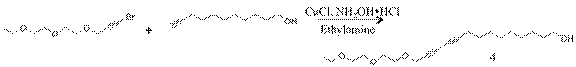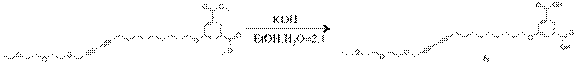Reports: G7
46485-G7 Origin of Mechanochromism in Polydiacetylene Compound
Polydiacetylene is known to exist in two separate phases distinguished by drastic changes to its optical properties. While the particular uniqueness of polydiacetylene has driven its recent popularity, there remains insufficient understanding about the actual mechanism by which it changes between phases. It is believed that the two phases exist as separate conformations to the bond structure of the conjugated polydiacetylene backbone.
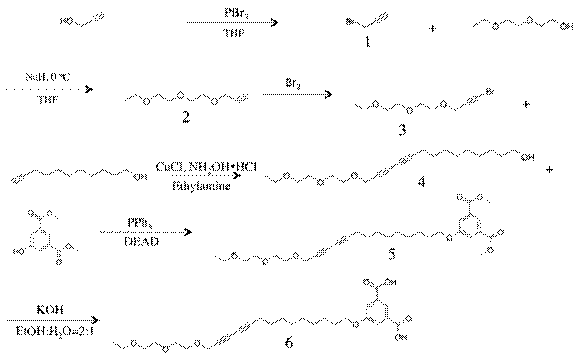
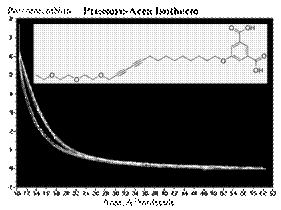
For the second year of the project, we have investigated new diacetylene monomer having precise amphiphilic property. We observed completely reversible pressure-area isotherms of the molecule on a Langmuir-Blodgett trough. The chemical structure and synthesis of the molecule and intermediates are illustrated in the figure below.
Propargyl bromide(1): To a 5g (0.044mol) of propargyl alcohol was added with 2mL of PBr3 in anhydrous THF under 10oC. The reaction mixture was stirred for 48 hours in an ice water bath at temperatures below 10oC. The solution was extracted with petroleum ether, washed with di-water and dried over anhydrous MgSO4, and filtered.
3-(2-(2-ethoxyethoxy)ethoxy)prop-1-yne(2): To a 3.00g (0.022mmol) of diethylene glycol monoethyl ether in 50ml of THF was added 1.34g (0.055mmol) of NaH in small portion. The mixture was stirred for 30 min. at 0oC. To the reaction mixture 4.25g (0.035mmol) of propargyl bromide was added in small portion. The final reaction mixture was stirred 1 hr at 0oC and 3 hr at room temp. The resulting residue was evaporated and purified by silica gel column chromatography (Ethylacetate:Hexane=2:3) to give 3g of yellow liquid.
1-bromo-3-(2-(2-ethoxyethoxy)ethoxy)prop-1-yne(3): To a 14.35g (0.25 mmol) of potassium hydroxide in 100ml of ice di-water was added 5.84g (0.036mmol) of bromine was added dropwise by addition funnel and temperature was maintained to 0oC~5oC. The reaction mixture was stirred for 30 min. The resulting solution was extracted with ether and further purified with silica gel column chromatography (Ethylacetate:Hexane=2:3) to give a 2.5g of pale yellow liquid.
14-(2-(2-ethoxyethoxy)ethoxy)tetradeca-10,12-diyn-1-ol(4): To a 4.62g (0.27 mmol) of 10-undecin-1-ol in 50ml of methanol was added 54.33mg (0.54mmol) of CuCl and 123.8 mg (1.92 mmol) of NH2OH in 10 ml of aqueous ethylamine. The mixture solution was bubbled with argon for 15 min. To a reaction mixture 6.89g (0.27mmol) of brominated ethylne glycol monoethyl ether was added over 30 min. in 20 ml of methanol. The resulting reaction mixture was stirred 3 hr at room temp and 200 ml of di-water was added. The residue was extracted with ether and washed with saturated Na2CO3. The final resulting solution was dried with MgSO4 and further purified with column chromatography (ethylacetate:hexane=1:1) to give a 6.2g of yellow liquid.
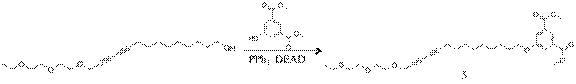
Dimethyl 5-((14-(2-(2-ethoxyethoxy)ethoxy)tetradeca-10,12-diyn-1-yl)oxy)isophthalate(5): To a 1.67g (7.97 mmol) of diethyl 5-hydroxyisophthalate in 200ml of dry ether under N2 was added 3.00g (8.86mmol) of diacetylene alcohol and 2.32g (8.86 mmol) of PPh3. To a reaction mixture 1.54g (8.86mmol) of diethyl-azodicarboxylate (DEAD) was added dropwise at -5oC. The resulting reaction mixture was stirred 8 hr at room temp. The residue was extracted with di-water and recrystalized in methanol. The final resulting solution was further purified with column chromatography (ethylacetate:hexane=1:2) to give a 1.65g of yellow liquid.
5-((14-(2-(2-ethoxyethoxy)ethoxy)tetradeca-10,12-diyn-1-yl)oxy)isophthalic acid(6): To a 1.65g (3.11 mmol) of diethyl 5-hydroxyisophthalated diacetylene monomer in 150ml of ethanol:H2O (2:1) was added 0.62mg (11.05mmol) of KOH. To a reaction mixture was refluxed for 4 hr at 80oC. Ether was removed by evaporation. To a resulting aqueous residue 5ml of HCl was added and stirred for additional 10 min. The final resulting solution was extracted with ether. The organic layer was dried with MgSO4 and was further purified with column chromatography (ethylacetate:hexane:MeOH=2:1:0.3) to give a 1.5g of yellow powder.
The reaction for above diacetylene structure 7 was almost same with the previous monomer. Undecinoic acid was used for diacetylene monomer instead of undecinoic alcohol and also 5-hydroxyisophthalic acid was used for the final isophthalic acid group.
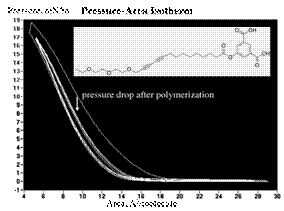
We completed the synthesis of compound 6 and 7 in the second year and the new diacetylene have shown a reversible pressure-area isotherm. Using the Langmuir method and the designed amphiphilic properties of the compound 6 and 7, we created monolayer of 6 and 7 at the air-water interface and compressed and released the monolayer by using the two barriers. The reversible pressure-area isotherms both molecules below show that the monolayers have amphiphilic property. Photo-polymerization of the resulting monolayers and the investigation on their phase transfer property are our next goal to achieve.
Besides the surfactant study described above we also investigate the effect of the analyte size on the mechanochromism of the polydiacetylenes (Donghwan Seo, Jinsang Kim Effect of the Molecular Size of Analytes on Polydiacetylene Chromism Adv. Funct. Mater. 2010 in press). In this study the pH chromism of polydiacetylenes (PDAs) is examined with respect to the molecular size and acidity of acid analytes, along with the alkyl spacer length of primary amine-functionalized diacetylene (DA) lipids. pH turns out to be an important parameter to charge amine headgroups of PDA but a change in pH does not necessarily result in PDA color change. The molecular size of acid analytes is identified as another factor that can produce a configurational change in PDA amine headgroups, followed by perturbation of the ene-yne conjugated backbone. In addition, the length of flexible alkyl spacer between the amine headgroup and the amide group of the diacetylene lipids is found to strongly affect the degree of PDA chromatic transition. The longer alkyl spacer shows a smaller chromatic transition from the blue phase to the red phase. The alkyl spacer seems to provide a certain degree of freedom to the amine headgroup, thus decreases the transfer of headgroup steric effects to the PDA backbone. These correlations found for PDA chromism are applied to the development of a system that colorimetrically detects diethyl phosphate (DEP), a degraded nerve agent simulant. PDA liposomes show a selective chromatic transition upon binding with DEP compared to other acid analytes.