Reports: G1
46285-G1 Nucleophilic Substitution/Functionalization Reactions of Transition-Metal Complexes at sp3 Carbon
Recently, transient vinyl-gold intermediates have been reported to participate in a variety of intramolecular trapping and rearrangement reactions.1-3 Carboauration of alkynes offers an attractive possibility for accessing vinyl-gold intermediates from readily available starting materials, however, the uncatalyzed intermolecular carborauration of alkynes requires multiple-week reaction times and Puddephatt reports it for only one substrate, bis(trifluoromethyl)acetylene.4, 5 A general intermolecular carboauration reaction would permit access to vinyl-gold intermediates for application in coupling and functionalization reactions for the synthesis of regiochemically and diasteromerically pure di- and tri-substituted olefins. As part of our ongoing work employing the unique reactivity of two metals to develop dual-catalyzed reactions,6,7,8 we developed a new Pd-catalyzed carborauration of alkynes that proceeds in 2 h at ambient temperature with complete regioselectivity and diastereoselectivity for the product of syn addition. This work was performed during the recent ACS-PRF funding period.
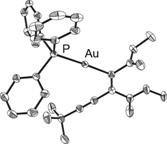
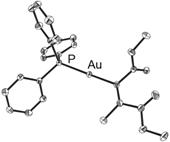
The utility of the Pd-catalyzed carboauration reaction is illustrated by a range of gold starting materials (i.e., sp, sp2, sp3, electron poor and electron rich aryl; Table 1).9 In all cases, a reaction selectivity for syn addition yielded diastereomerically pure products as confirmed by 1H NMR spectroscopy and by X-ray crystallographic studies of 8 and 9 (Figure 1). Syn addition is opposite to the selectivity reported by Toste for the uncatalyzed intramolecular carboauration of alkynes.10-12
Table 1. Scope of Pd-Catalyzed Carboauration Reaction
a relative to mesitylene internal standard. b 15 mol % Pd, 48 h. c 2.0 equiv DMAD, 30 min.
Figure 1. ORTEP structures of 8 and 9. Thermal ellipsoids shown at 50% probability. Hydrogen atoms omitted for clarity.
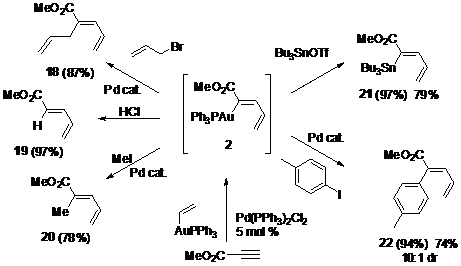
We next explored the reactivity of the vinyl-gold products. A range of Pd and Au cross-coupling reactions proceeded in high yield with retention of configuration, opening synthetic access to trisubstituted olefins with complete regio- and diastereocontrol (Scheme 1, 18, 20, 22). In each functionalization reaction, crude vinyl-gold 2 was employed without isolation, and the residual Pd catalyst from the carboauration reaction remained viable for the subsequent cross-coupling step, establishing a one-pot tandem carboauration/functionalization protocol. For example, after 2 h, addition of allyl bromide to crude 2 in the same solution used for the carboauration reaction produced allylated product 18 (87%, two steps), presumably through a p-allyl palladium intermediate. These new cross-coupling reactions proceed through a fully characterized homogeneous gold intermediate, removing ambiguity pertaining to the nature of the reactive gold species.13
The strongly electrophilic reagents HCl and Bu3SnOTf trapped vinyl gold 2 in the absence of a Pd catalyst (both 97%, two steps). In these cases, purified 2 displayed similar reactivity to crude 2. Mechanistic work in this paper suggests a reinterpretation of the catalytic carbostannylation reaction we previously reported, to include the intermediacy of a vinyl-gold compound.7
Scheme 1. One pot carboauration/functionalization.a
a Two-step 1H NMR yields relative to mesitylene internal standard.
In conclusion, we have developed a diastereo- and regioselective Pd-catalyzed carboauration reaction of alkynes. This reaction provides synthetic access to fully characterized vinyl-gold intermediates. These intermediates participate in a series of intermolecular palladium-catalyzed cross-coupling reactions and electrophilic trapping reactions to produce di- and trisubstituted olefins with high diastereoselectivity and absolute regioselectivity. In a broader sense, the reactions reported herein provide a demonstration of reactivity available to Au/Pd systems and expand the synthetic utility of vinyl-gold intermediates. Application of these principles to the development of new Au- and Pd- cocatalyzed reactions (i.e., catalytic in both metals rather than just palladium) are ongoing in our laboratory. Given the prolific role of both Pd and Au separately in organic synthesis, more “combined concepts” are likely forthcoming as this fundamental understanding grows. Application of these principles to the development of new Au- and Pd- cocatalyzed reactions (i.e., catalytic in both metals rather than just palladium) are ongoing in our laboratory. Given the prolific role of both Pd and Au separately in organic synthesis, more “combined concepts” are likely forthcoming as this fundamental understanding grows.
References
1. Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395.
2. Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180.
3. Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410.
4. Johnson, A.; Puddephatt, R. J. J. Chem. Soc., Dalton Trans. 1978, 115, 980.
5. For a photochemical carboauration of tetrafluoroethylene, see: Mitchell, C. M.; Stone, F. G. A. J. Chem. Soc., Dalton Trans. 1972, 102.
6. Duschenk, A.; Kirsch, S. F. Angew. Chem., Int. Ed. 2008, 47, 5703.
7. Shi, Y.; Peterson, S. M.; Haberaecker, W. W., III; Blum, S. A. J. Am. Chem. Soc. 2008, 130, 2168.
8. For a review on bimetallic catalysis by late transition metals, see: van den Beuken, E. K.; Feringa, B. L. Tetrahedron 1998, 54, 12985.
9. Formation of 15 (vida infra) was competitive with formation of 9, accounting for the lower yield of product 9.
10. Kennedy-Smith, J. J.; Staben, S. T.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 4526.
11. A gold-hydride complex was recently shown to participate in anti-hydroauration of DMAD: Tsui, E. Y.; Müeller, P.; Sadighi, J. P. Angew. Chem., Int. Ed. 2008, 47, 8937.
12. The absence of reentry of products 1-9 into the catalytic cycle could derive from changes in steric and electronic factors relative to the starting vinyl gold complex.
13. Isolated examples of Au/Pd cross-coupling reactions have been reported: Jones, L. A.; Sanz, S.; Laguna, M. Catal. Today 2007, 122, 403.