Reports: G1
45899-G1 Brønsted Acid-Catalyzed Asymmetric Additions to Imines
1. Catalytic Asymmetric Alcohol Additions
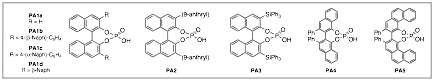
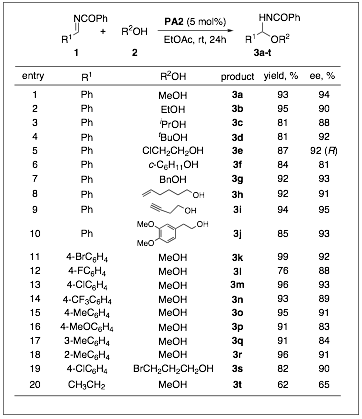
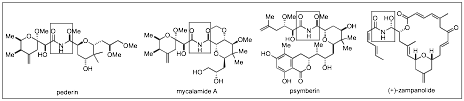
We have completed our studies into the addition of alcohol nucleophiles to imines during the grant period. This work was started in the prior period but was finished and published late in 2008. In our work we have shown that the chiral phosphoric acid-catalyzed (Figure 1) addition is very general in alcohol, with very high asymmetric excess being found (Table 1). The impact of this work could be to realize natural product synthesis (Scheme 2) and we have begun this work recently.
Figure 1. Chiral Phosphoric Acid Catalysts.
Note: Please refer back to Figure 1 throughout the document for catalyst structures.
Table 1: Catalytic Asymmetric Alcohol Additions to Imines.
Figure 2: Select Natural Product with Chiral Hemiaminal Core Structures.
2. The Highly Enantioselective Hydrogenation of Enamides.
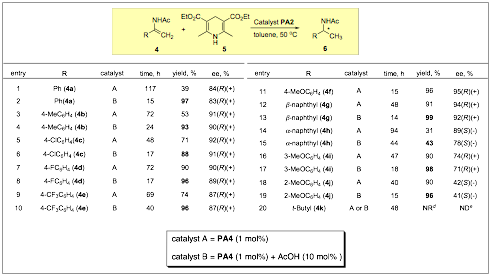
Although enantioselective hydrogenation of ketones, imines, and enamides is a developed metal-catalyzed field with highly selective methods we believed that chiral phosphoric acids could possibly impact the field through the development of complimentary methods that do not require metal-based catalyst. We found that catalyst PA2 was again a good activator in the case of these different substrates. We were disappointed to eventually find the limits rather quickly (R can only be aryl substituents and substitution elsewhere on the enamides is not tolerated) on the reactions scope but we were able to achieve reasonably good selectivities along with an achiral co-acid effect that lowered the catalyst loading to 1 mol% (Table 2).
Table 2: Catalytic Enantioselective Hydrogenation of Enamides.
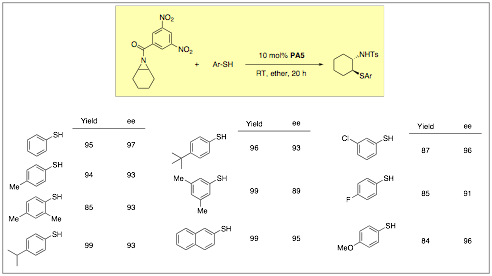
3. Chiral Phosphoric Acid-Catalyzed Ring-Opening of meso-Aziridines with Thiophenols.
The catalytic asymmetric ring-opening of aziridines is an important, actively pursued area of research. Recently, we have discovered that chiral Brznsted acids can catalyze the ring opening of meso-aziridines with azide to provide a 1,2-diamine precursor in high enantiomeric excess and reported this in the last grant year. These desymmetrization reactions occur at room temperature and with moderate to high yield. We have now increased our scope in terms of the nucleophile and have shown thiophenols can again ring open meso-aziridines with high enantioselectivty using PA5. This work was just recently reported and ongoing related studies are underway based upon this work.
Table 3: Brznsted Acid-Catalyzed Ring-Opening Desymmetrization of meso-Aziridines with Thiols.